Kinetic Particle Theory
Kinetic Particle Theory:
* describes the states of matter
* explains the differences in the properties of solids, liquids and gases
* explains the changes in state of matter
States that all matter is made up of tiny particles that are in (1) constant random motion and (2) constantly collide with each other.
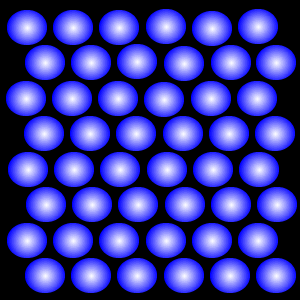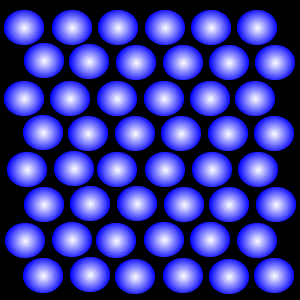
Solid

Liquid

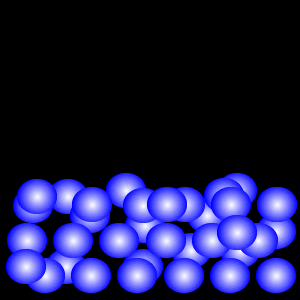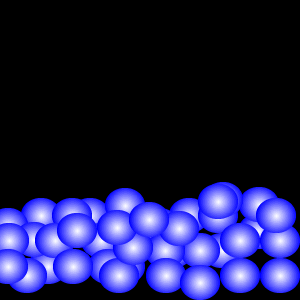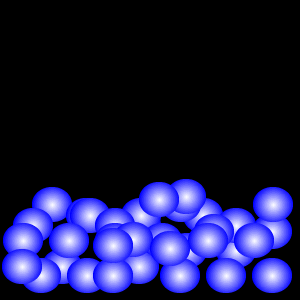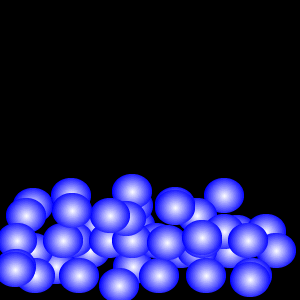
Gas

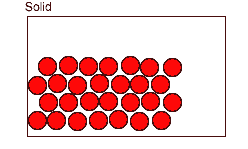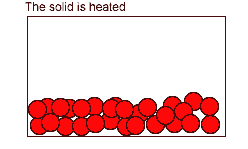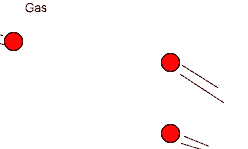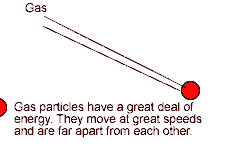
The Solid State
Why does a solid have a fixed shape?
According to the kinetic particle theory, the particles of a solid
* are closely packed in an orderly manner
* are held together by very strong forces of attraction
* have enough kinetic energy to only vibrate and rotate about their fixed positions
* cannot move about freely
Hence, a solid has a fixed shape.
Why does a solid have a fixed volume?
A solid cannot be compressed since its particles are already very close to one another, giving it a fixed volume
The Liquid State
There is more space between the particles of a liquid than that of a solid.
Why does a liquid not have a fixed shape?
According to the kinetic particle theory, the particles of a liquid:
* are arranged in a disorderly manner
* have weaker forces of attraction than the particles of a solid
* have more kinetic energy than particles of the same substance in the solid state, and are not held in fixed positions
* can move freely throughout the liquid
Hence, a liquid has no fixed shape.
Why does a liquid have a fixed volume?
The particles of a liquid are further away from one another than the particles of a solid. However, the liquid particles are still packed quite closely together. Thus a liquid cannot be compressed and has a fixed volume.
The Gaseous State
Why does a gas not have a fixed shape?
According to the kinetic particle theory, the particles of a gas
* are spread far apart from one another
* have weaker forces of attraction than the particles of a liquid
* have a lot of kinetic energy and are not held in fixed positions
* can move about rapidly in any direction
Thus a gas has no fixed shape.
Why does a gas not have a fixed volume?
The particles of a gas have a lot more space between them as compared to that of a liquid or solid. This allows the gas to be easily compressed when pressure is applied. Since a gas can be compressed, it has no fixed volume.
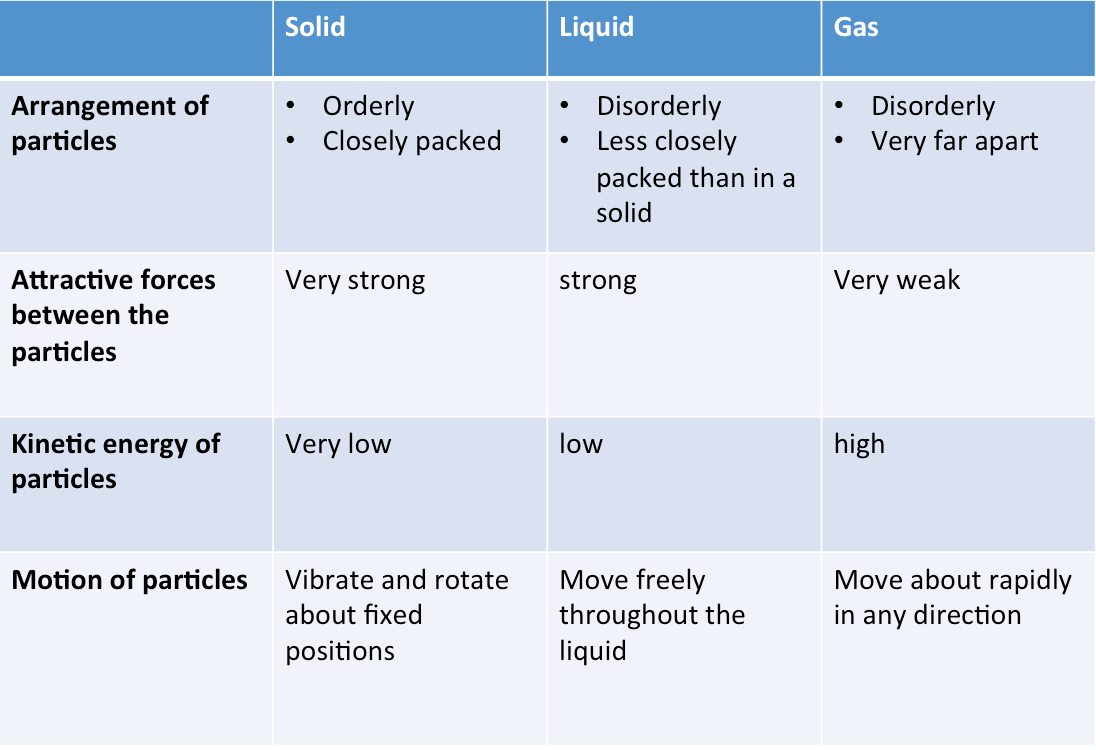
Summary
The table below is a summary of the properties of solids, liquids and gases.
Changes in State of Matter
Matter can change from one state to another. According to the kinetic particle theory, particles of matter are in constant motion -- they have kinetic energy. Gases have the most energy, followed by liquids. Solids have the least energy among the three states.
When matter is heated or cooled, the heat energy taken in or given out causes the kinetic energy of the particles to change. As a result, there is a change in state. Changes in state are reversible.
During a change in state of matter, heat energy is converted into kinetic energy of the particles of matter.
Melting
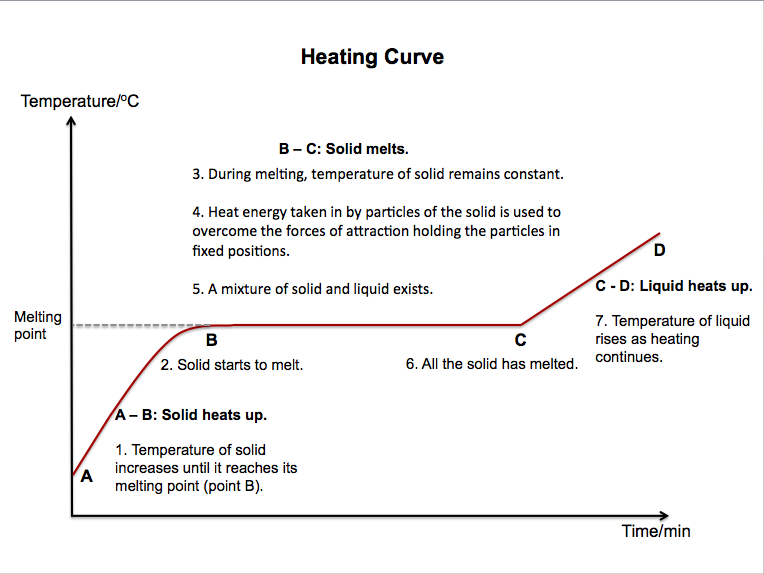
Melting is the process by which a substance changes from a solid to a liquid. The temperature at which a solid becomes a liquid is called its melting point.
What happens to the particles of a solid that is heated until it melts?
* Heat energy is absorbed by the particles of the solid.
* The heat energy is converted to kinetic energy.
* The particles start to vibrate faster about their fixed positions.
* When the temperature is high enough, the vibrations of the particles become sufficient to overcome the forces of attraction between them.
* The particles begin to break away from their fixed positions.
* When the particles are no longer in their fixed positions, the substance has become a liquid.
* The particles can move freely throughout the liquid.
A heating curve can be used to show how the temperature of a solid changes as it is heated to its melting point and beyond.
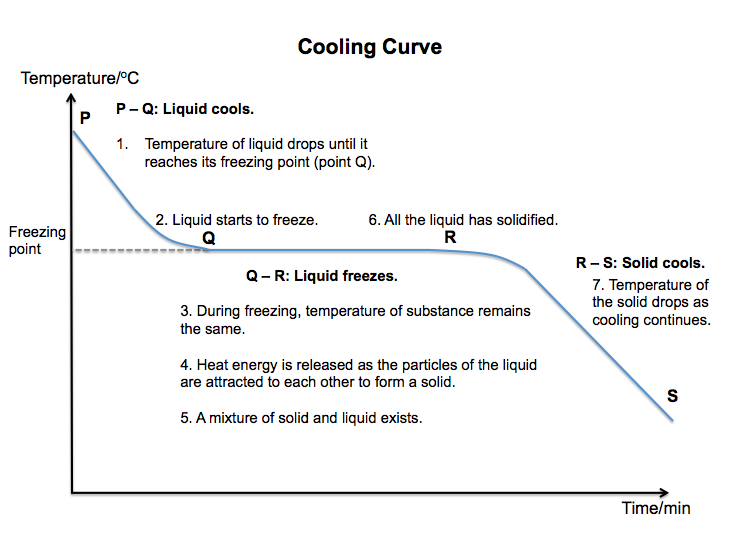
Freezing
Freezing is the process by which a substance changes from a liquid to a solid. The temperature at which a liquid becomes a solid is called its freezing point. A pure substance melts and freezes at the same temperature.
What happens to the particles of a liquid that is cooled until it freezes?
* Energy is given out by the particles of the liquid.
* The particles lose kinetic energy and begin to move more slowly.
* When the temperature is low enough, the particles no longer have enough energy to move freely.
* The particles start to settle into fixed positions.
* When all the particles have settled into fixed positions, the substance has become a solid.
* The particles can only vibrate about their fixed positions.
A cooling curve can be used to show how the temperature of a pure liquid changes as it is cooled to its freezing point and beyond.
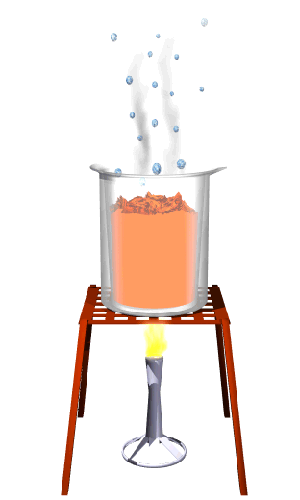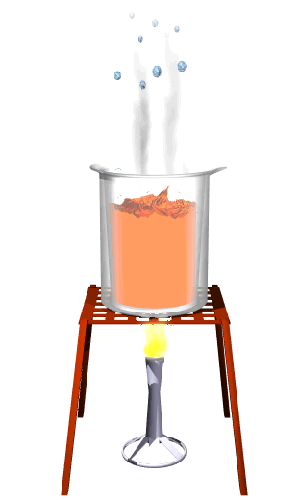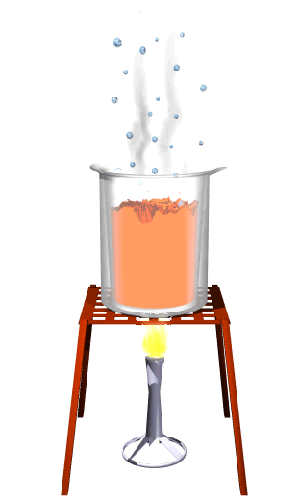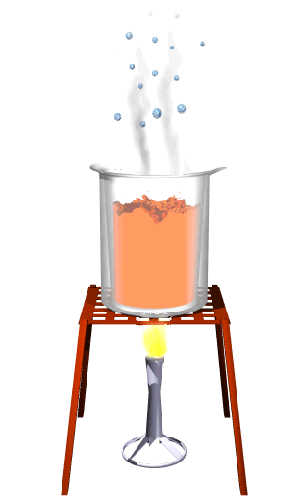
Boiling
Boiling is the process by which a substance changes from a liquid to a gas at the boiling temperature of the substance. The temperature at which a liquid boils is called its boiling point.
What happens to the particles of a liquid that is heated until it boils?
* Heat energy is absorbed by the particles of the liquid.
* The heat energy is converted into kinetic energy.
* The particles start to move faster as the temperature rises.
* When the temperature is high enough, the particles have enough energy to overcome the forces of attraction holding them together.
* The particles are now spread far apart.
* The substance is now a gas.
* The particles can move about in any direction.
Evaporation
Evaporation is the process by which a liquid changes to a gas at temperatures lower than its boiling point. Evaporation occurs because some particles have enough energy to escape as a gas from the surface of the liquid.
Liquids that evaporate quickly at room temperature are called volatile liquids. They usually have boiling points just above room temperature. Petrol and perfumes are examples of volatile liquids.
How is evaporation different from boiling?
Both boiling and evaporation involve a liquid changing to a gas, but boiling is different from evaporation in three ways. The table below shows the difference between boiling and evaporation.
Condensation
Condensation is the process by which a gas changes to a liquid. When water vapour touches a cold surface, condensation occurs and liquid water is obtained.
Condensation can be thought as the reverse of boiling. Heat energy is given out by the gas particles during condensation. As the temperature drops, the gas particles lose energy and move more slowly. Eventually, the movement of the particles becomes slow enough for the gas to change to a liquid.
Sublimation
Sublimation is the process by which a solid changes directly to a gas without going through the liquid state. It occurs because particles at the surface of the solid have enough energy to break away from the solid and escapes as a gas.
Examples of solids that sublime:
* Solid carbon dioxide (also known as dry ice)
* Ammonium chloride
* Iodine
Atoms, Molecules and Ions
Atom
An atom is made up of a nucleus (protons and neutrons) and orbitals with electrons.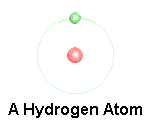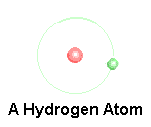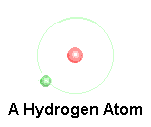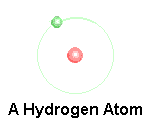
Molecule
A molecule is two or more atoms chemically bonded together.
Ion
An ion is an atom or molecule with an electric charge due to loss or gain of electrons