Measuring Mass
The S.I. unit for mass is the kilogram (kg). Smaller masses are measured in grams (g). The tonne (t) is used to measures the masses of heavy objects like vehicles.
* 1kg = 1000g
* 1 tonne (t) = 1000kg
The mass of a substance is measured with a beam balance or an electronic balance. An electronic balance, with an accuracy of up to
Measuring Time
The S.I. unit for time is the second (s). Other units include the minute (min) and the hour (h).
* 1h = 60 min
*1 min = 60 s
A stopwatch is used to measure time in the laboratory. The accuracy of an analogue stopwatch is
Measuring Temperature
The S.I. unit for temperature is the kelvin (K). Another commonly used unit is the degree Celcius (
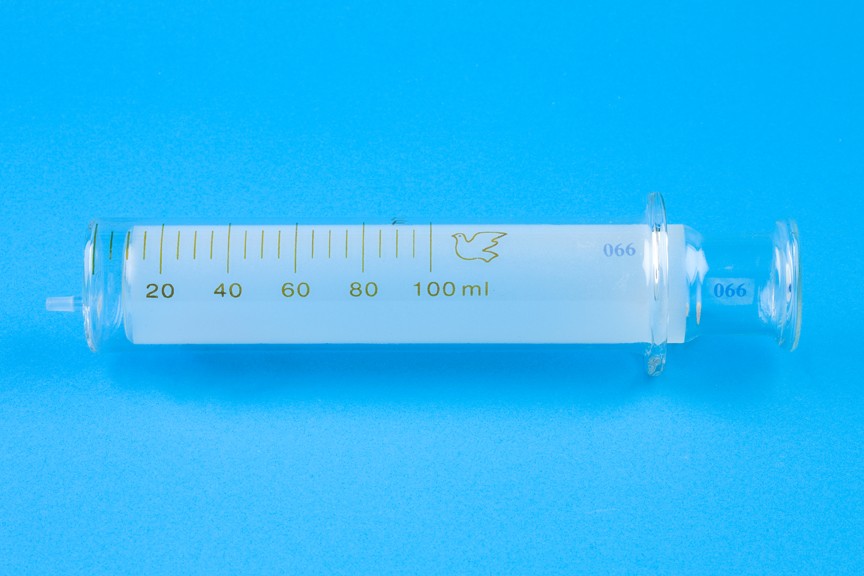
Measuring Volume
The S.I. unit for volume is the cubic metre (m
Measuring Cylinder
* Measures to the nearest 0.5cm
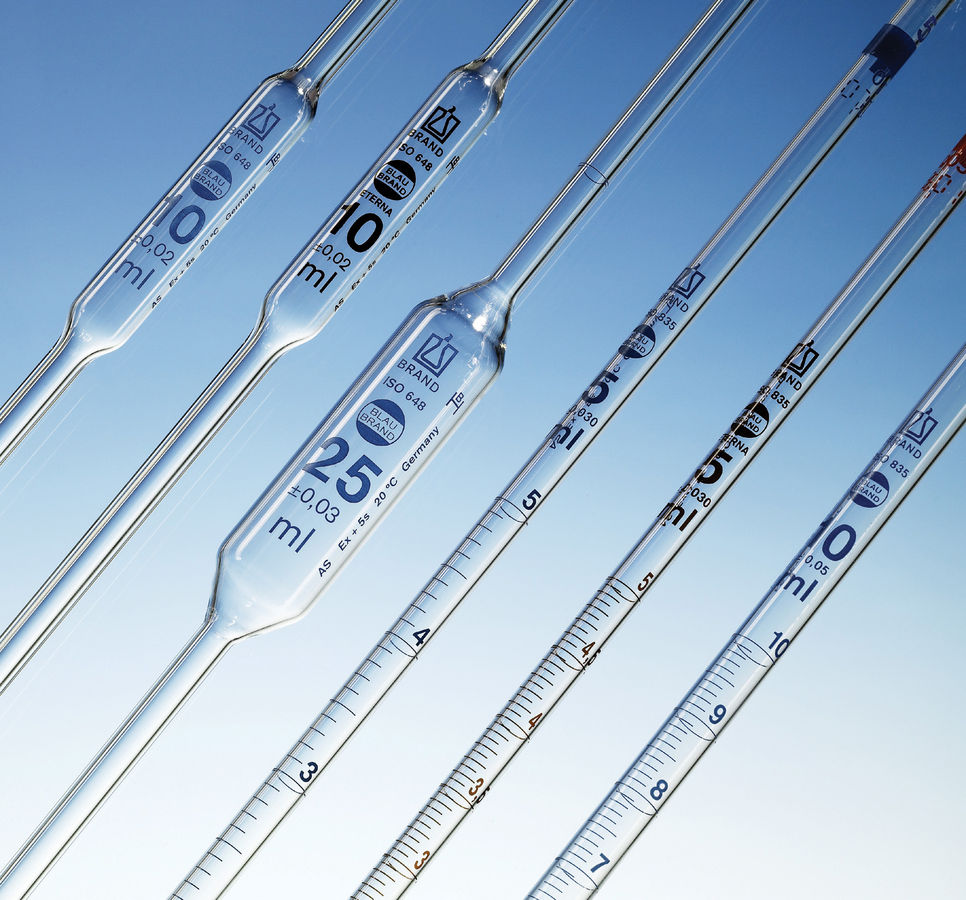
Burette
* Accurately measures out the volume of a liquid to the nearest 0.05cm
Pipette
* Accurately measures out fixed volumes of liquids, e.g. 20.0 cm
Difference Between a Mixture and a Pure Substance
A mixture is made up of two or more substances that are not chemically combined.
A pure substance is made up of one single element or compound. It is not mixed with any other substance.
Filtration
Filtration is used to separate insoluble solid particles from a liquid.
For example, sand can be separated from a mixture of sand and water by filtration. The residue can be collected by drying on a piece of filter paper, while the filtrate can be collected in the conical flask.
Evaporation to Dryness
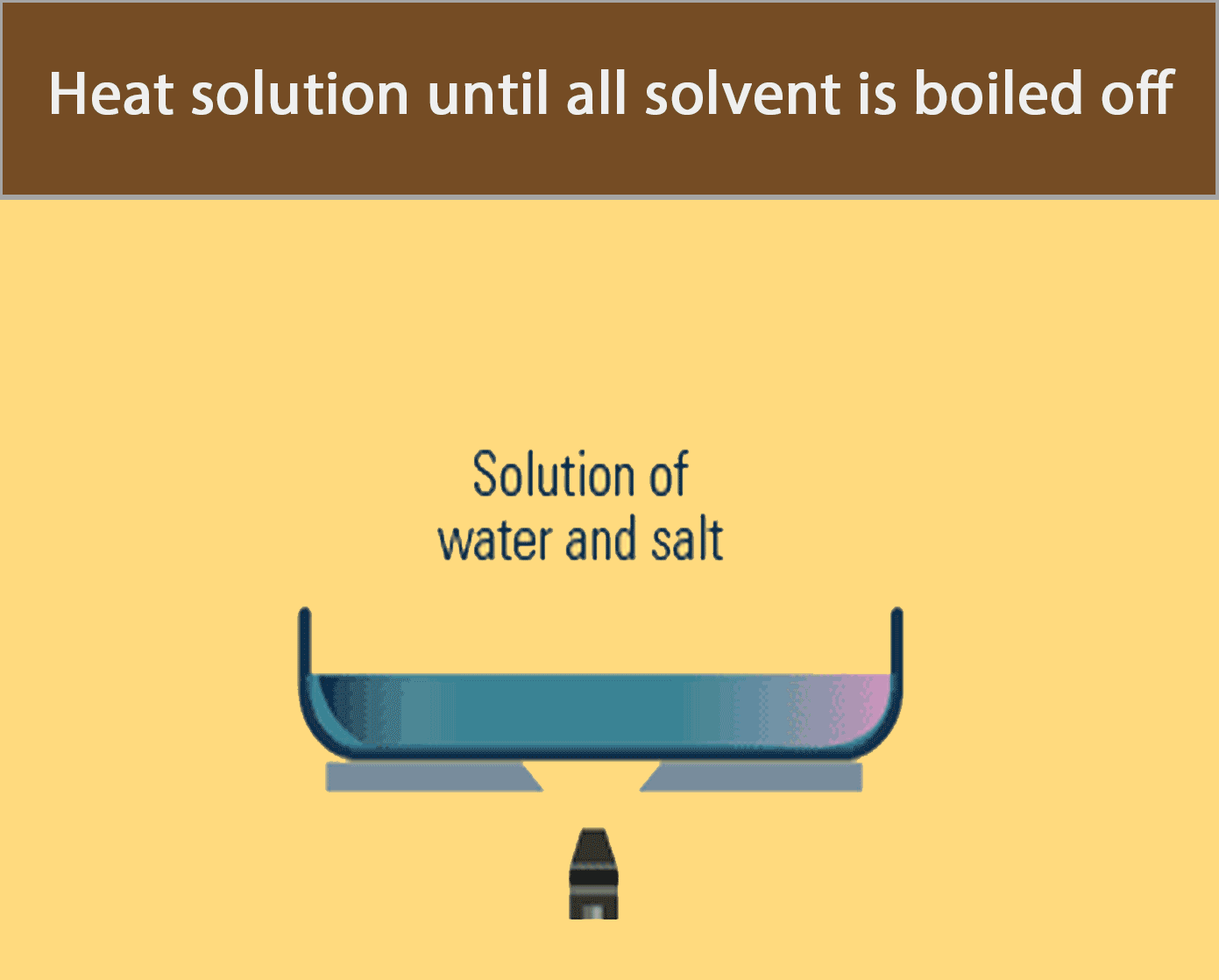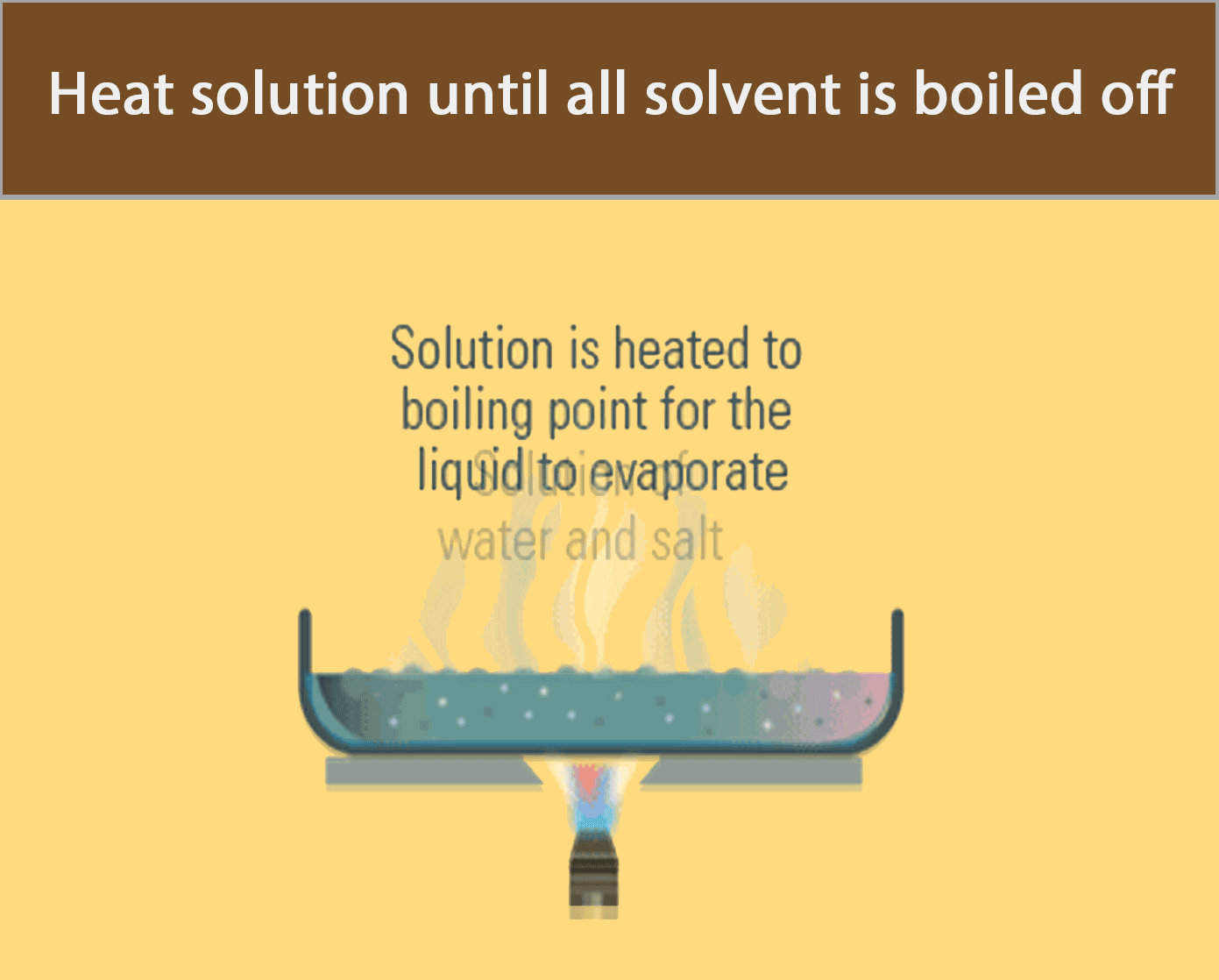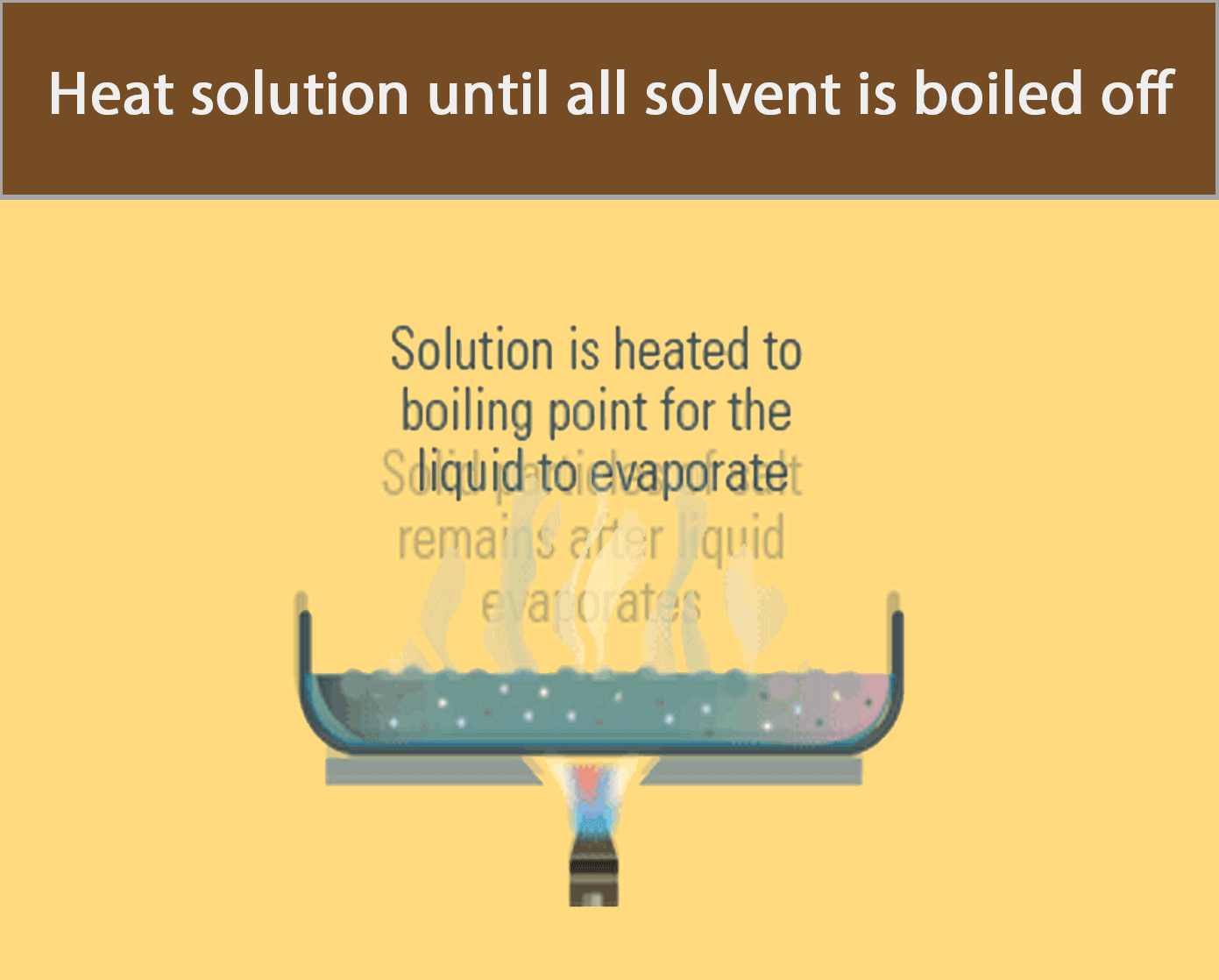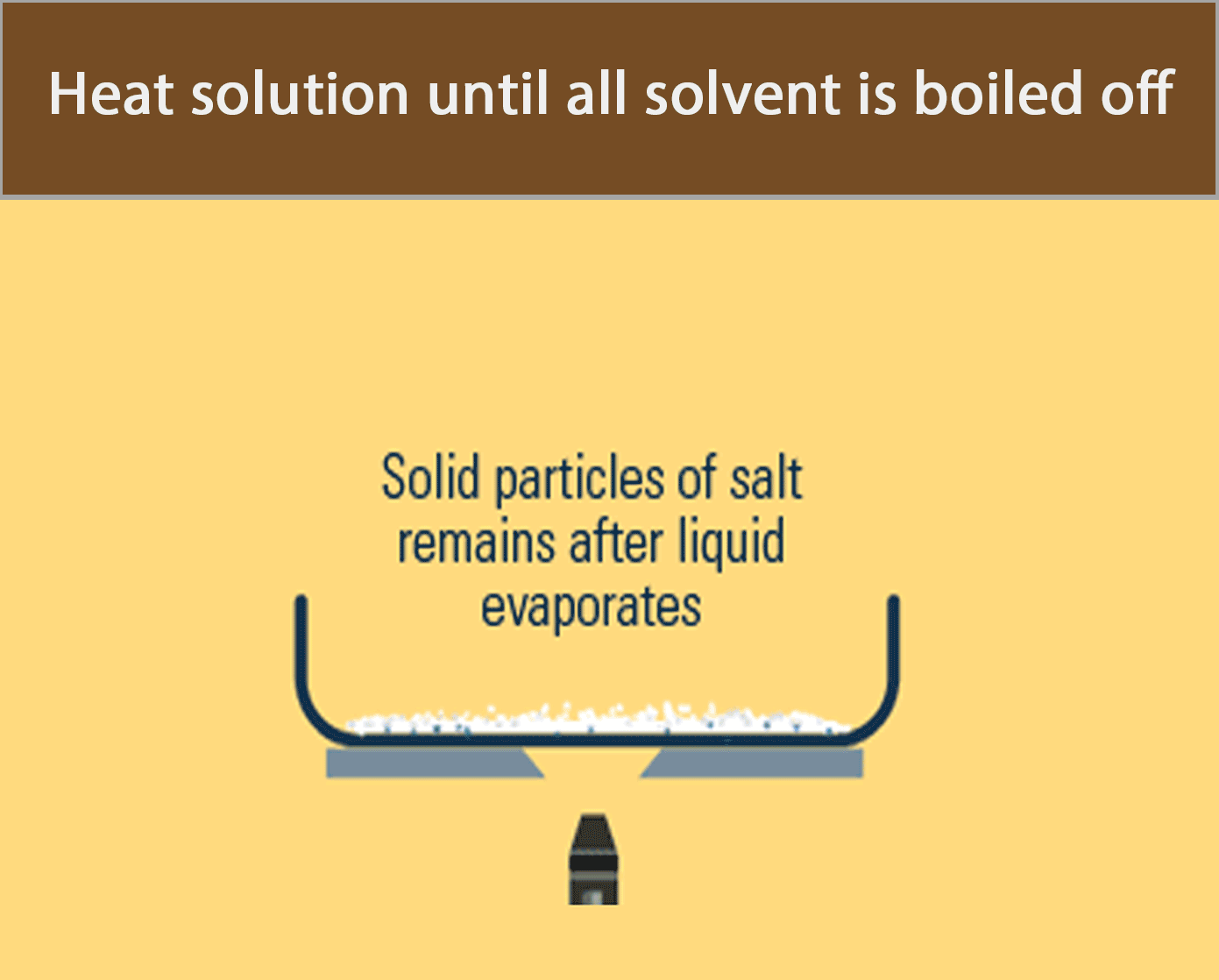
Evaporation to dryness is used to obtain a soluble solid from a solution by heating the solution until all the water has boiled off.
The apparatus in the figure below can be used to recover solid salt from a salt solution by evaporation to dryness.
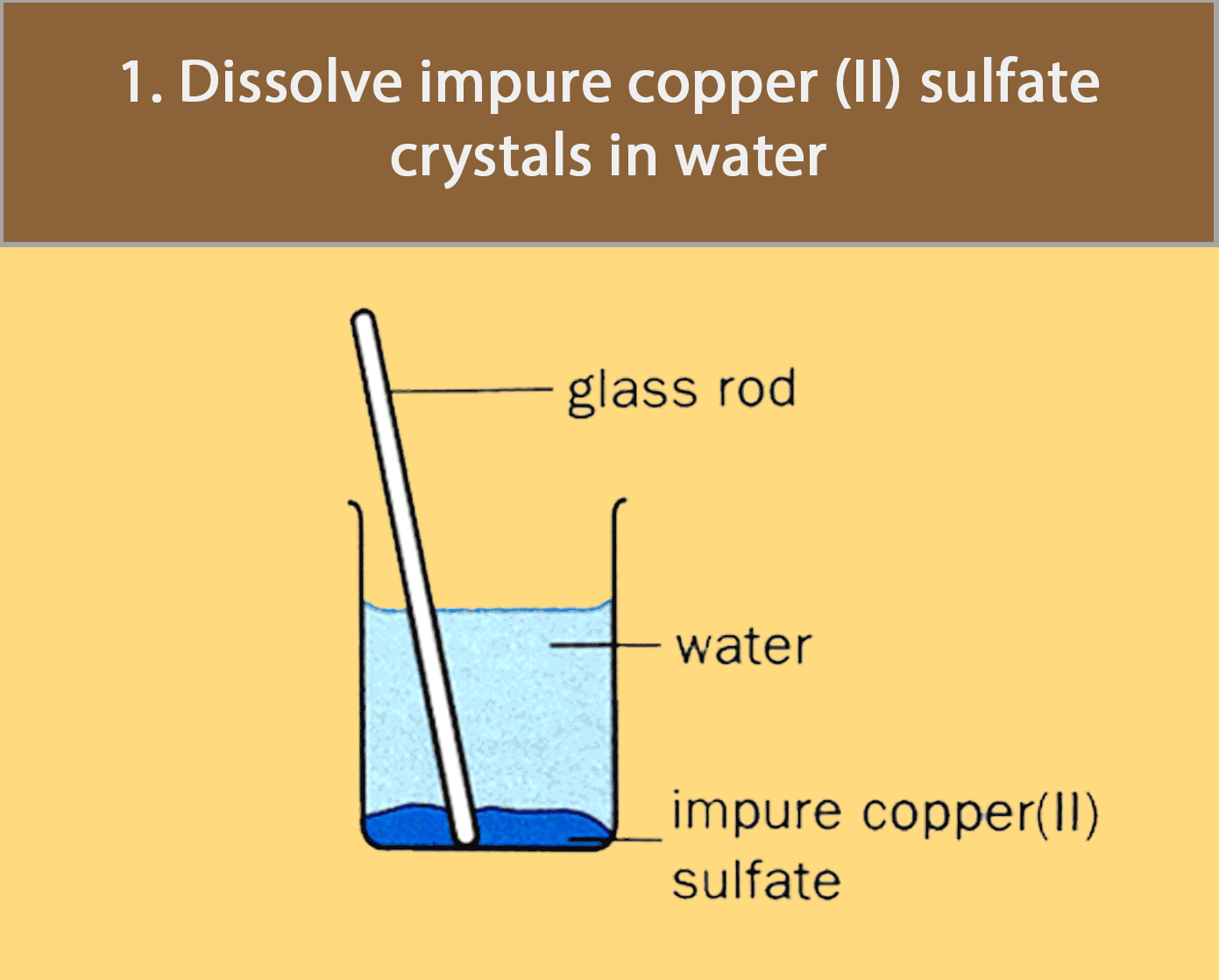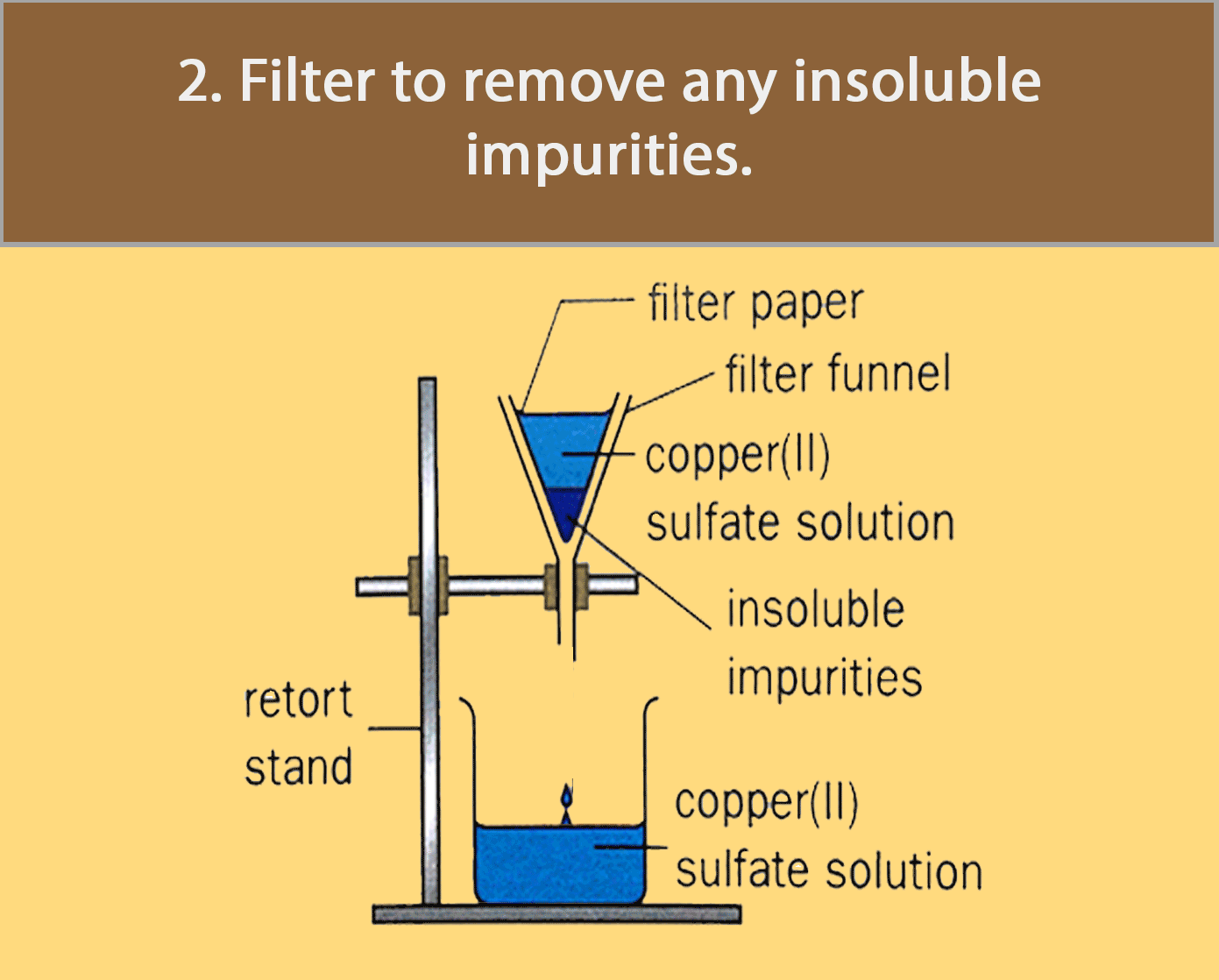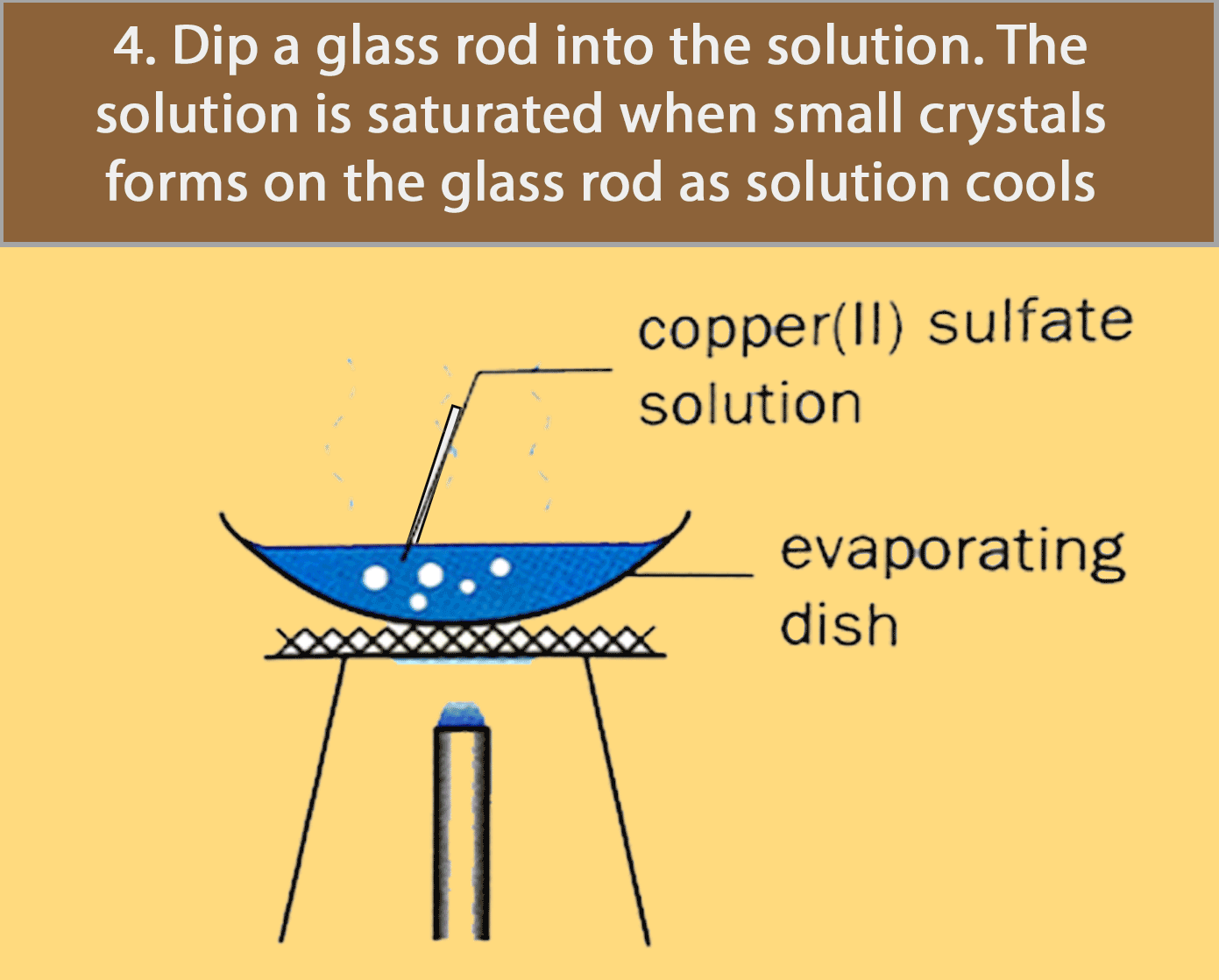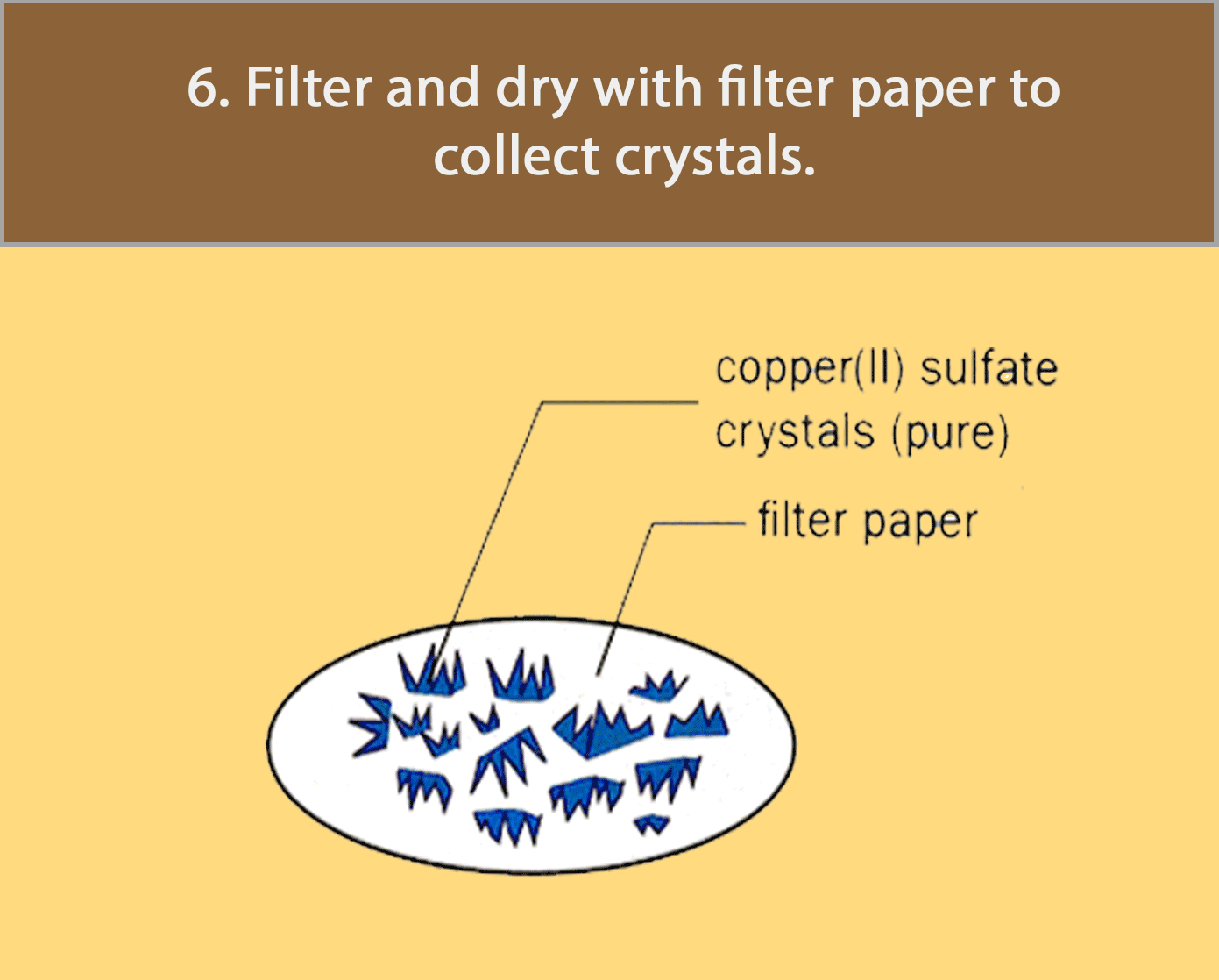
Crystallisation
Crystallisation is used for obtaining a pure solid sample from its solution.
Many substances decompose (break down to form simpler substances) when they are heated strongly. Most crystals, such as copper (II) sulfate crystals, give off water to become powders when heated. For such substances, evaporation to dryness is not a good method of separation and purification.
The figure below shows how pure copper (II) sulfate crystals are obtained by crystallisation.
Sublimation
Sublimation is used to separate a solid that sublimes from one that does not.
Some common substances that undergo sublimation upon heating are:
- dry ice (solid carbon dioxide)
- iodine crystals
- naphthalene (mothballs)
- camphor
- *ammonium chloride
Note: ammonium chloride does not actually undergo sublimation upon heating. It decomposes and forms ammonia gas and hydrogen chloride gas.
The video below shows the sublimation of iodine.
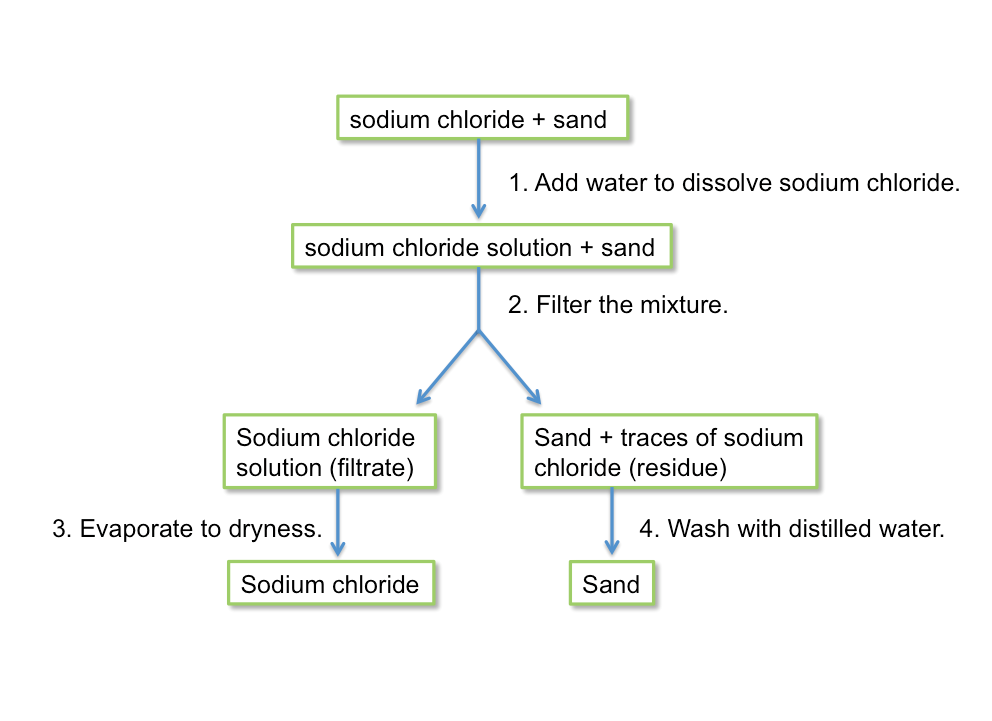
Dissolving and Filtration
To separate a mixture of two solids, we use a solvent in which only one solid is soluble. A solvent is a liquid that dissolves a substance (which is the solute).
Different solids dissolve in different solvents. Some commonly used solvents are water and ethanol.
An example of separating solids by dissolving in a suitable solvent followed by filtration is shown below.
Using a Magnet
A magnet can be used to separate a magnetic substance from a non-magnetic substance.
Examples of magnetic metals:
- iron
- nickel
- cobalt
The video below shows how a magnet can be used to separate iron from sand.
Simple Distillation
Simple distillation is used to separate a pure solvent (liquid) from a solution.
Distillation is the process of boiling a liquid and condensing the vapour. The figure below shows the apparatus used for simple distillation .
There are several procedures that have to be noted when setting up the distillation apparatus. Click to the last slide to see the procedures.
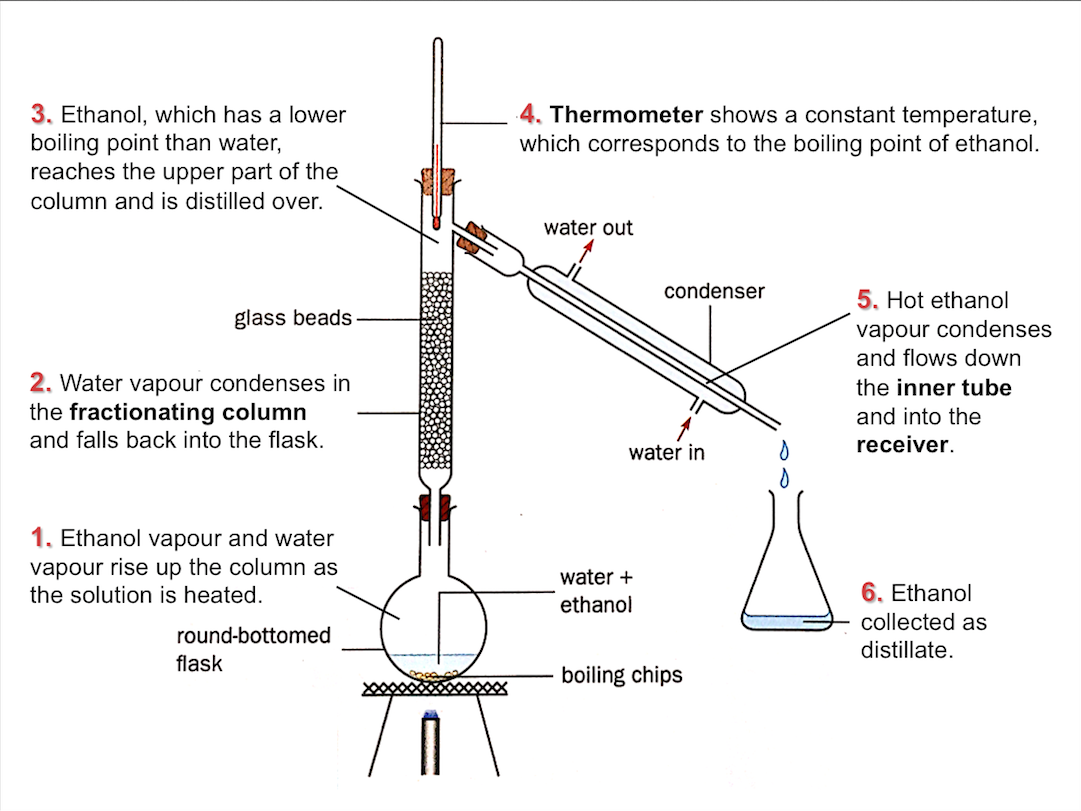
Fractional Distillation
Fractional distillation is used to separate a mixture of miscible liquids with different boiling points.
During fractional distillation,
- the liquid with the lowest boiling point distills over first
- the vapours of liquids with higher boiling point condense along the fractionating column and fall back into the round-bottomed flask
The figure below shows how ethanol can be separated from water via fractional distillation.
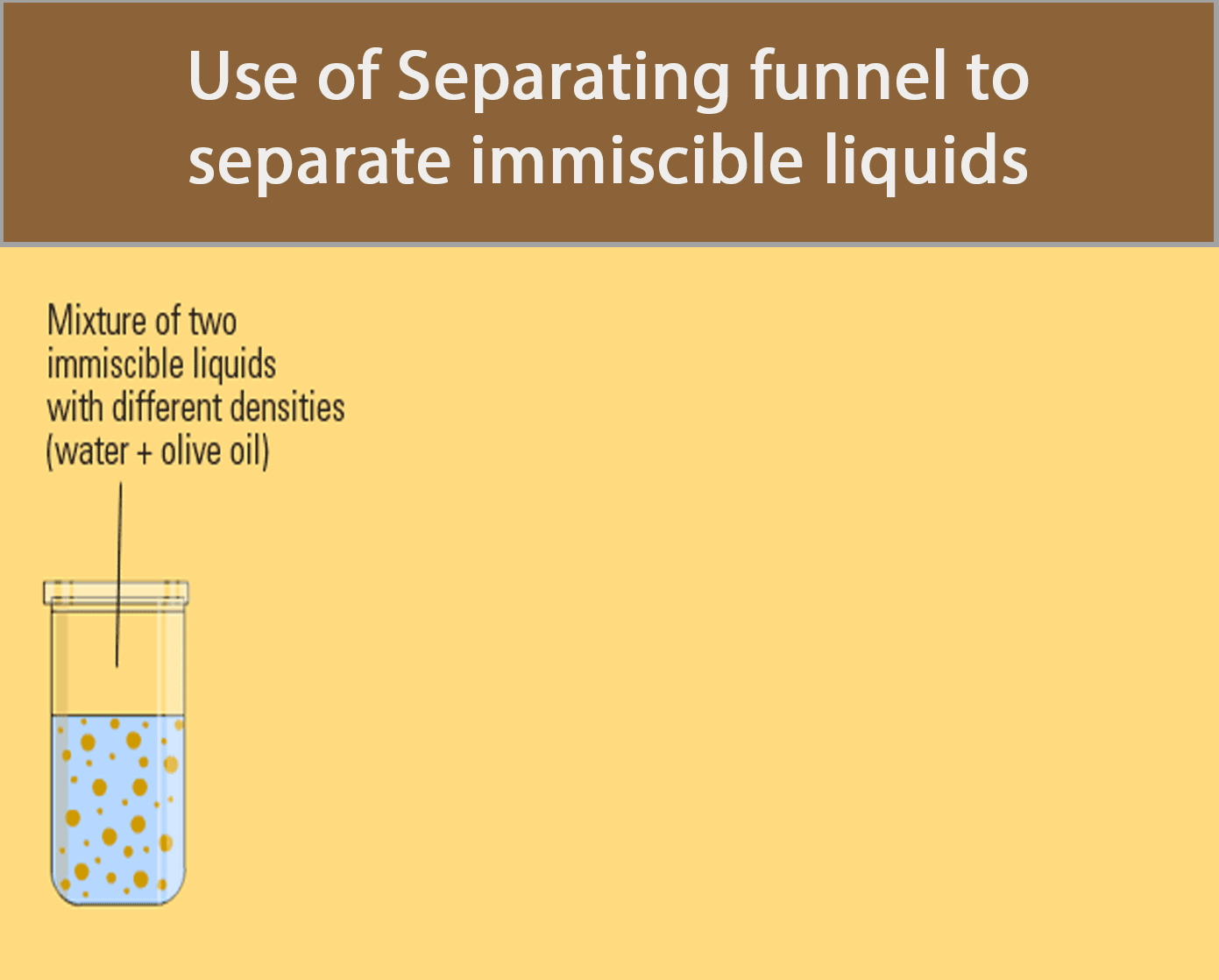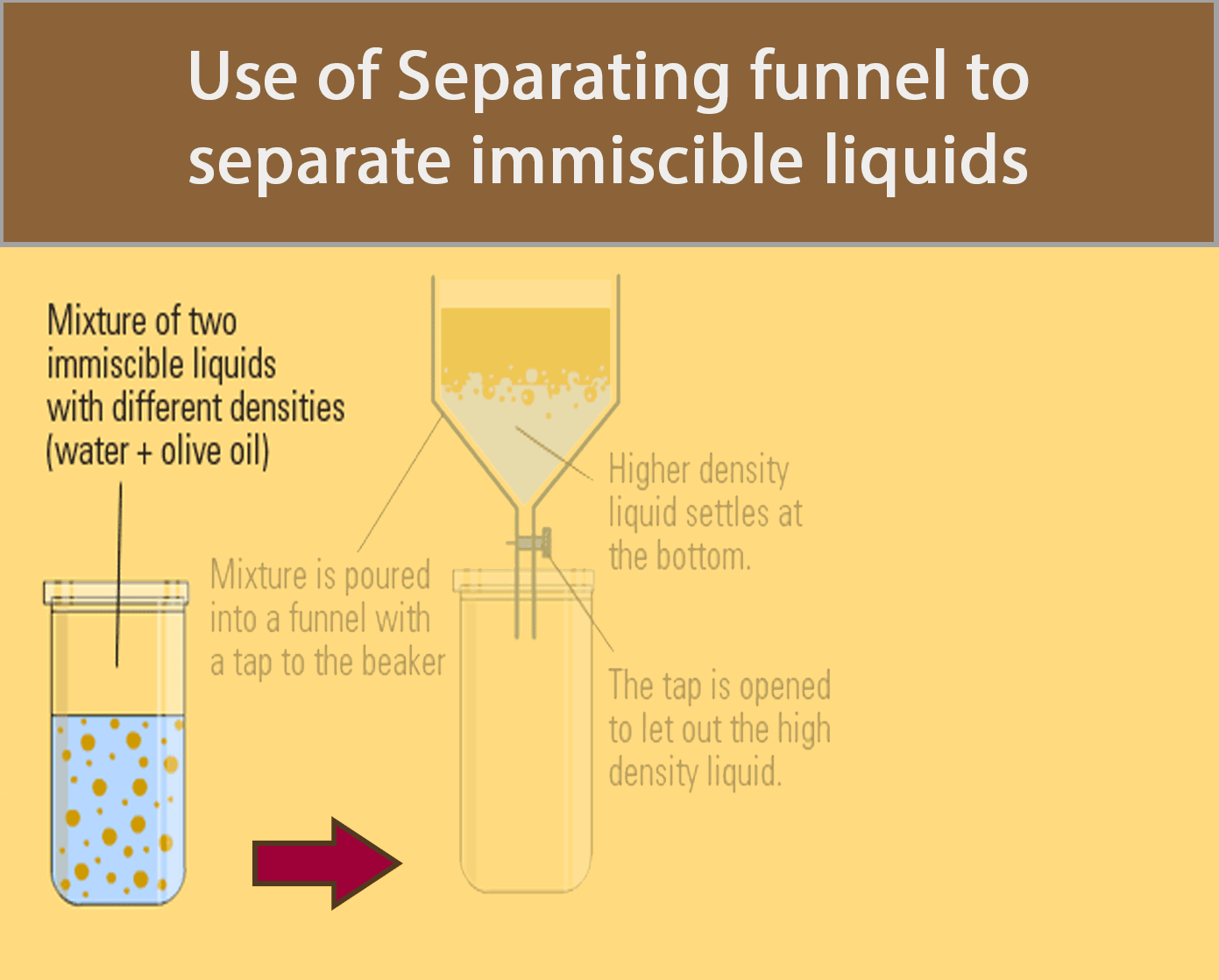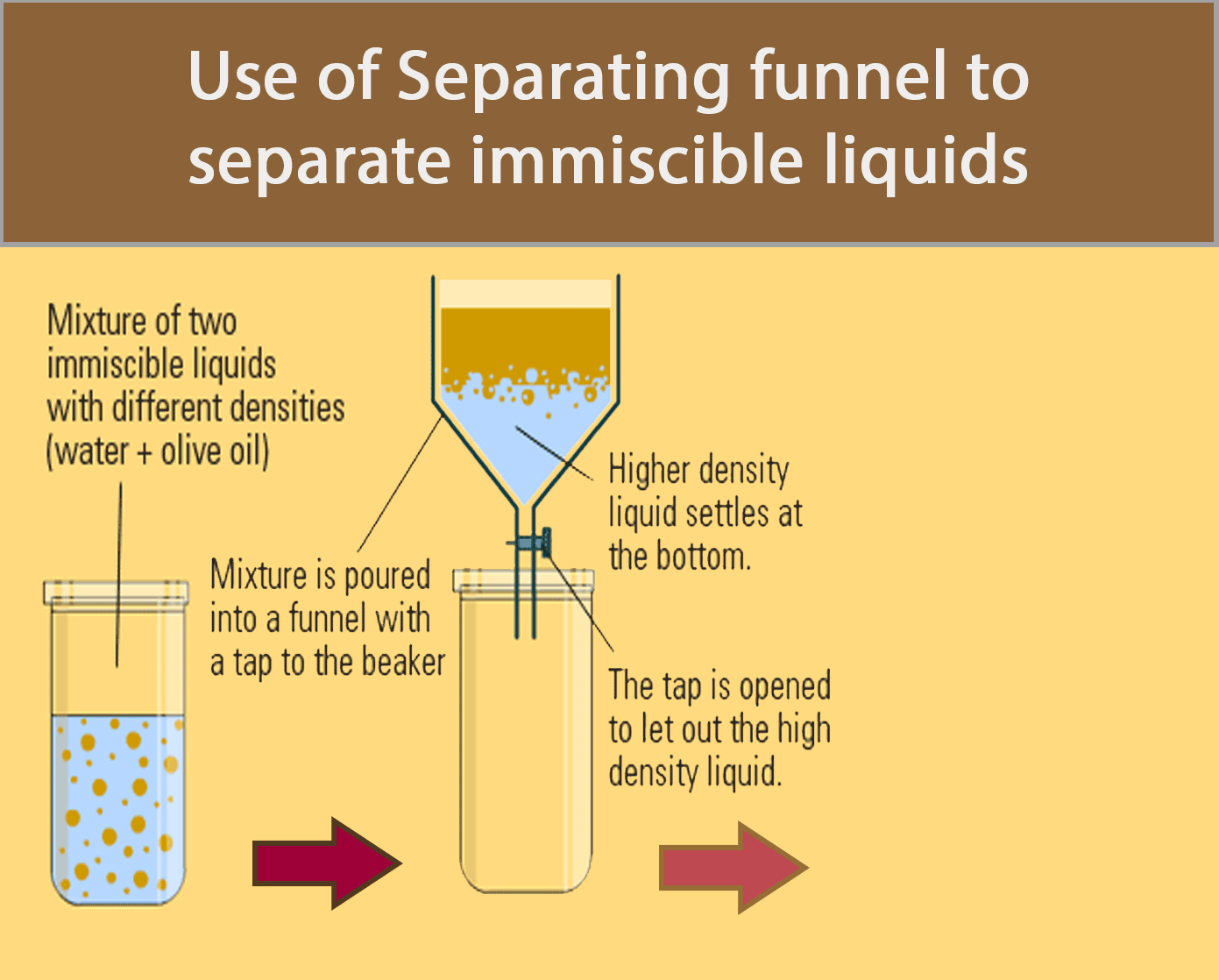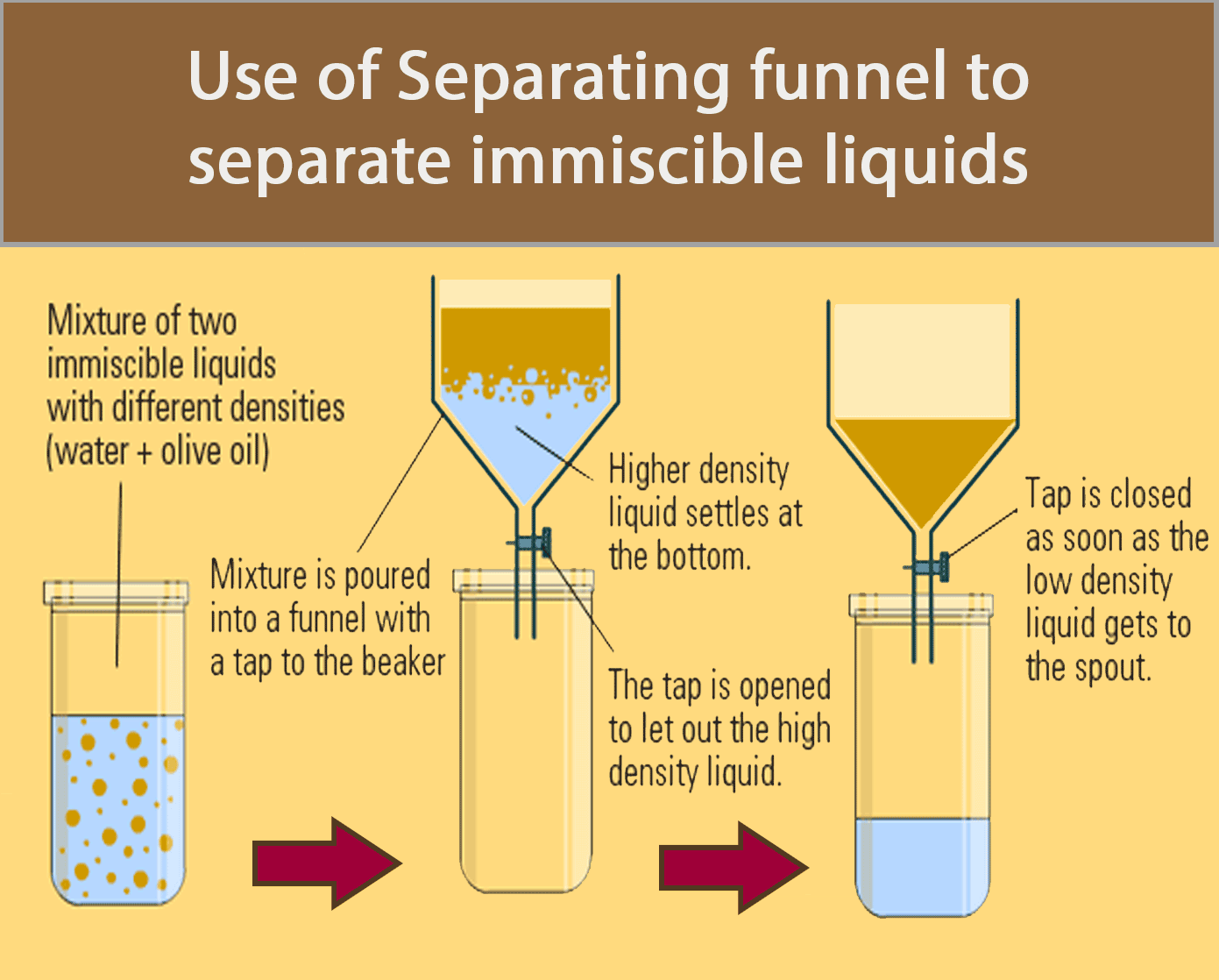
Separating Funnel
A separating funnel can be used to separate immiscible liquids.
The procedure below shows how a separating funnel is used to separate oil and water.
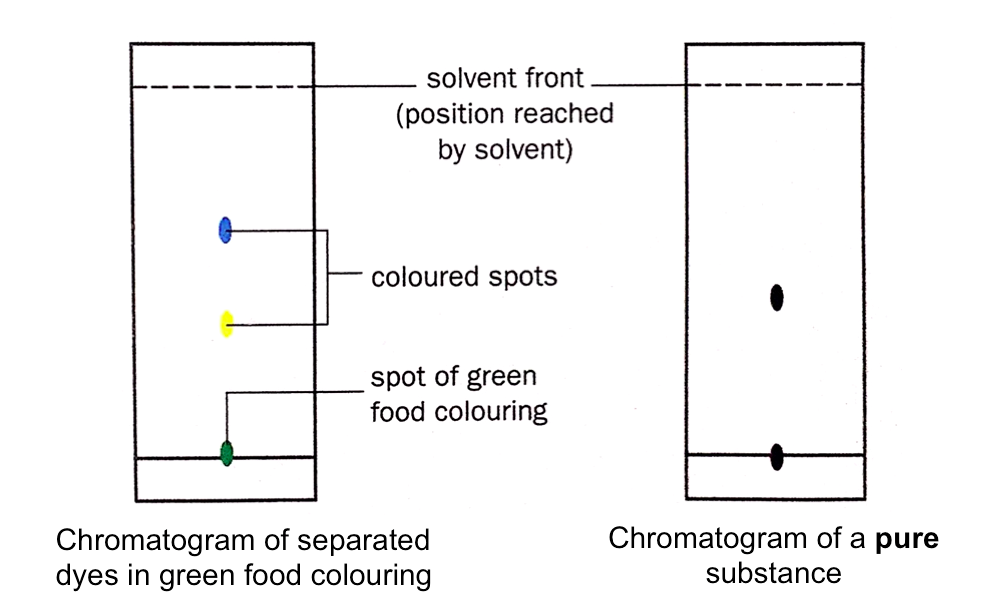
Paper Chromatography
Chromatography is the method of separating two or more components that dissolve in the same solvent.
For example, chromatography can be used to separate the dyes in food colouring. In the example below, paper chromatography will be used to separate the dyes in green food colouring.
Interpreting the Result of Paper Chromatography
The chromatography paper with the separated components is called a chromatogram.
The resulting chromatogram of the experiment above shows that the green food colouring is a mixture of two dyes. On the other hand, a pure substance is made up of only one dye and gives only one spot on a chromatogram.
Identical dyes produce spots at the same height and in the same colour on the chromatogram when the same solvent is used.
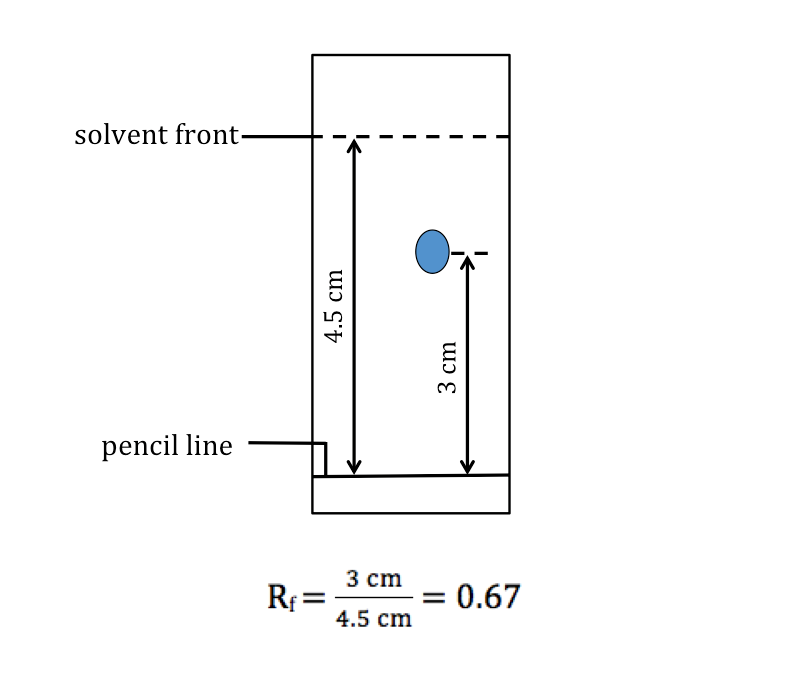
[Supplementary] Rf Values
How far the solvent front and substance travel up the chromatogram depends on how long the experiment is allowed to run. However, the ratio between the distance travelled by the substance and the distance travelled by the solvent is a constant, as long as chromatography is carried out under the same conditions (i.e. same solvent and same temperature). This ratio is called the rentention factor or Rf value of the substance.
Rf value = (distance travelled by the substance)/(distance travelled by the solvent)
[Supplementary] Measurement of Rf values
The figure below shows how Rf values are measured.
[Supplementary] Chromatography of Colourless Substances
When running a chromatogram of colourless substances such as amino acids, a locating agent is sprayed on the chromatogram. This causes the colourless substances to show up as coloured spots.
The video below summarises the key points of paper chromatography and explains the use of a locating agent (from 2:25).