Chemical Changes
Chemical changes take place on the molecular level.
A chemical change produces a new substance.
Examples of chemical changes include combustion (burning), cooking an egg, rusting of an iron pan, and mixing hydrochloric acid and sodium hydroxide to make salt and water.
Physical Change
Physical changes are concerned with energy and states of matter.
A physical change does not produce a new substance.
Changes in state or phase (melting, freezing, vaporization, condensation, sublimation) are physical changes.
Examples of physical changes include crushing a can, melting an ice cube, and breaking a bottle.
Difference between chemical and physical change
In a nutshell, a chemical change produces a new substance, while a physical change does not. A material may change shapes or forms while undergoing a physical change, but no chemical reactions occur and no new compounds are produced.
What is an element?
An element is a pure substance that cannot be broken down into two or more simpler substances by chemical processes.
Carbon, hydrogen and oxygen are examples of elements. They cannot be broken down further into simpler substances.
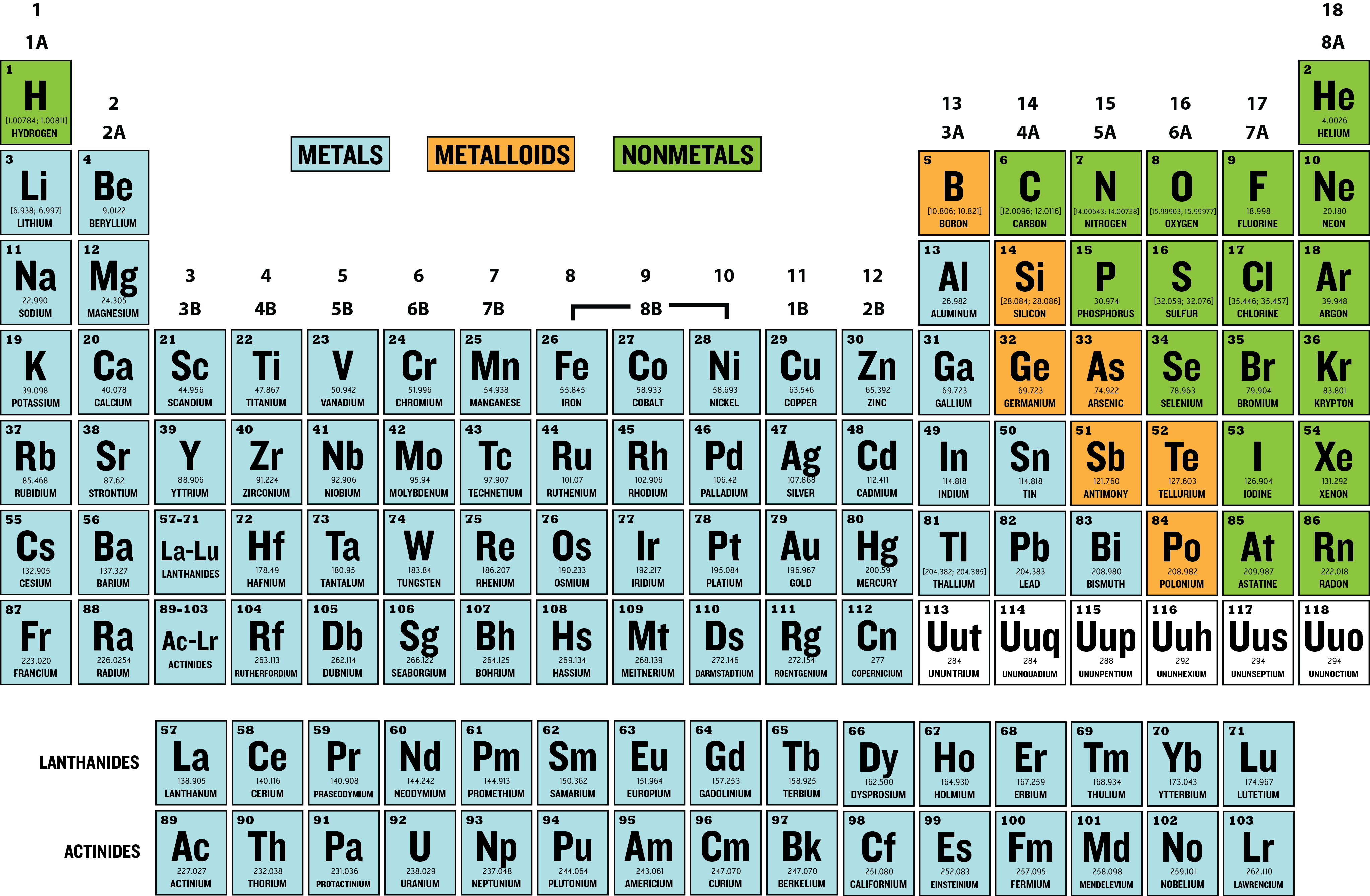
Classifying Elements
Elements are often classified based on their
- metallic and non-metallic properties
- physical states
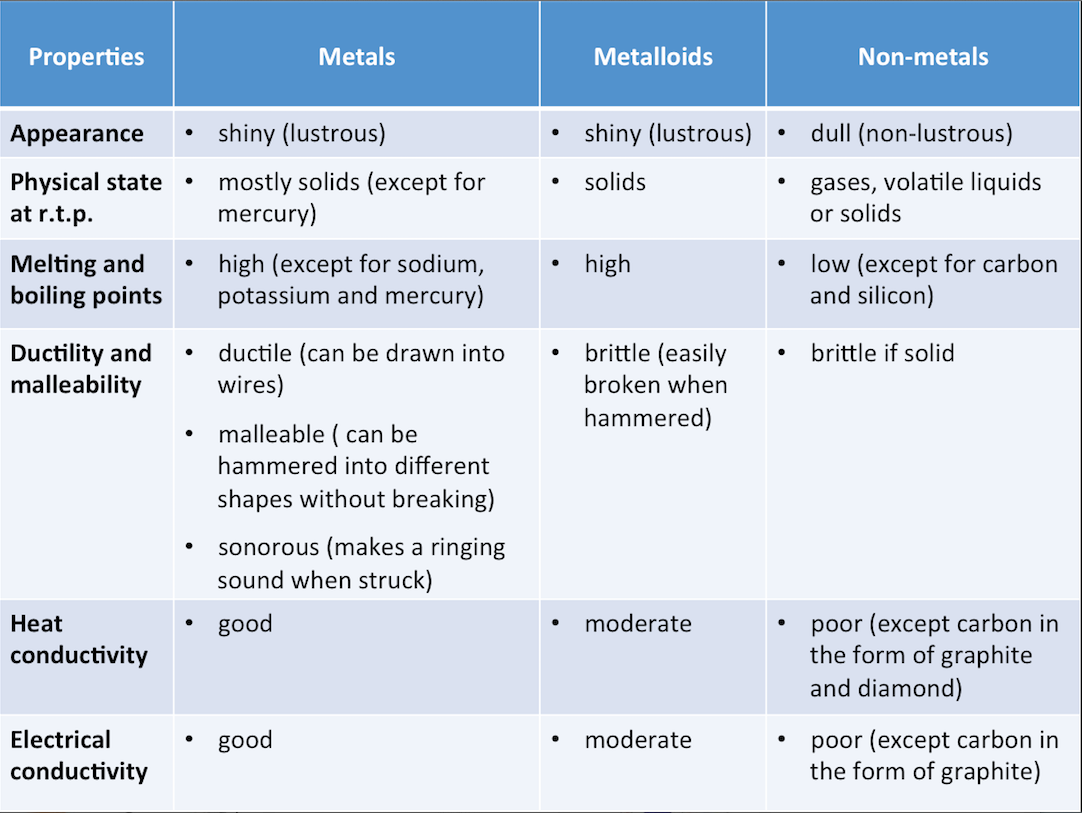
The table below shows the classification of elements by their metallic properties.
Atoms and Molecules
Elements can exist as atoms or molecules.
Atoms are the smallest particles of an element that have the chemical properties of that element. Each element contains only one type of atom.
Noble gases, such as helium, neon, argon, krypton, xenon and radon, are elements that exist as individual atoms. They are monatomic elements.
A molecule is a group of two or more atoms that are chemically combined.
Diatomic molecules are those that are formed by the combination of two atoms. Polyatomic molecules are those that contain three or more atoms.
Refer to the video below for a better understanding of atoms and molecules.
What is a compound?
A compound is a pure substance containing two or more elements that are chemically combined in a fixed ratio.
The video below explains how compounds are formed from their elements.
Composition of a Compound
A compound may be made up of molecules or ions.
An ion is an electrically charged particle that carries either positive or negative charge(s).
Formation of a Compound
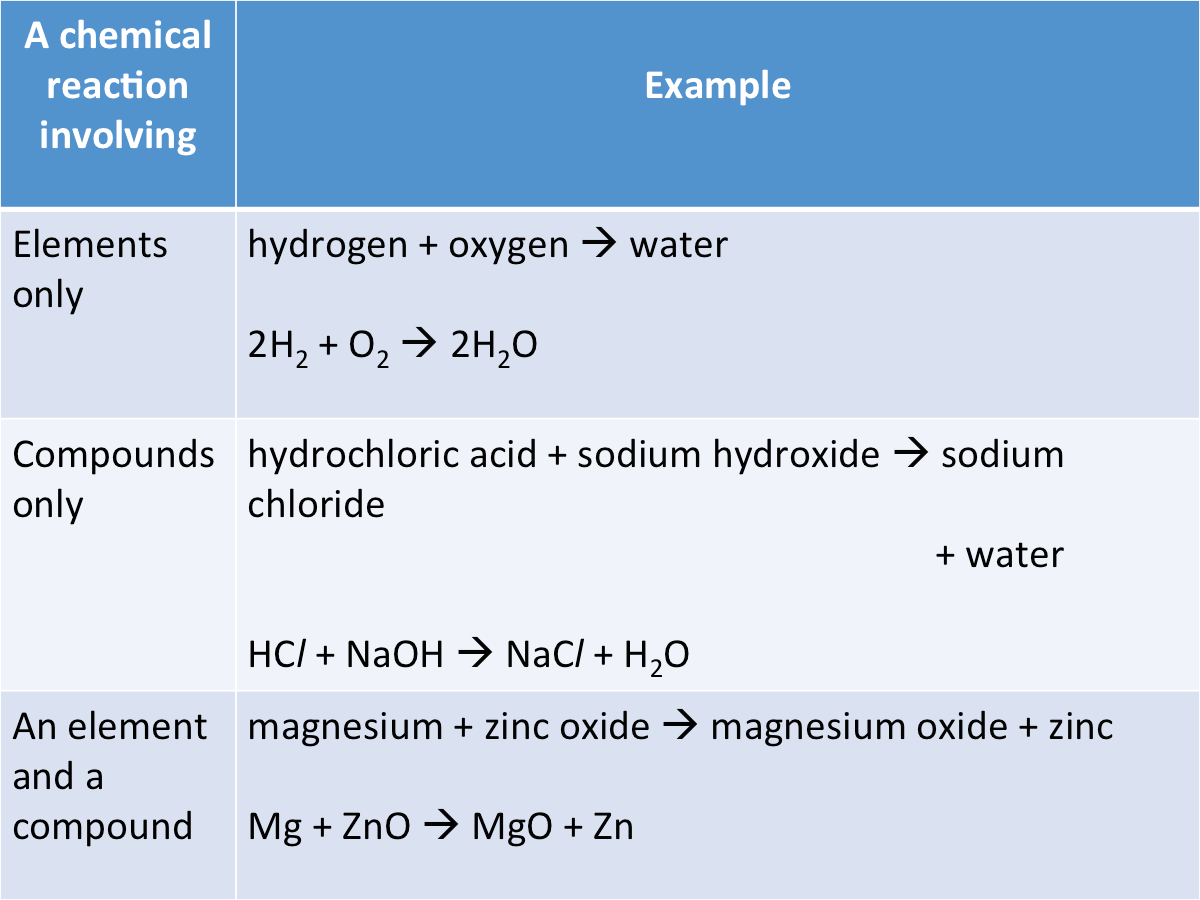
Compounds can be produced from a chemical reaction between elements only, compounds only or between elements and compounds.
Properties of a Compound
A compound has different properties from the elements that form it.
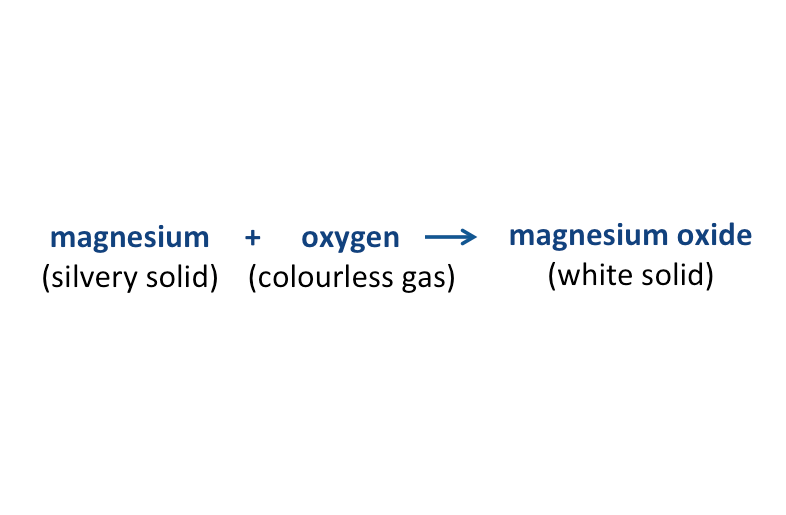
For example, magnesium burns in oxygen to form the compound magnesium oxide. Magnesium oxide has properties different from magnesium and oxygen.
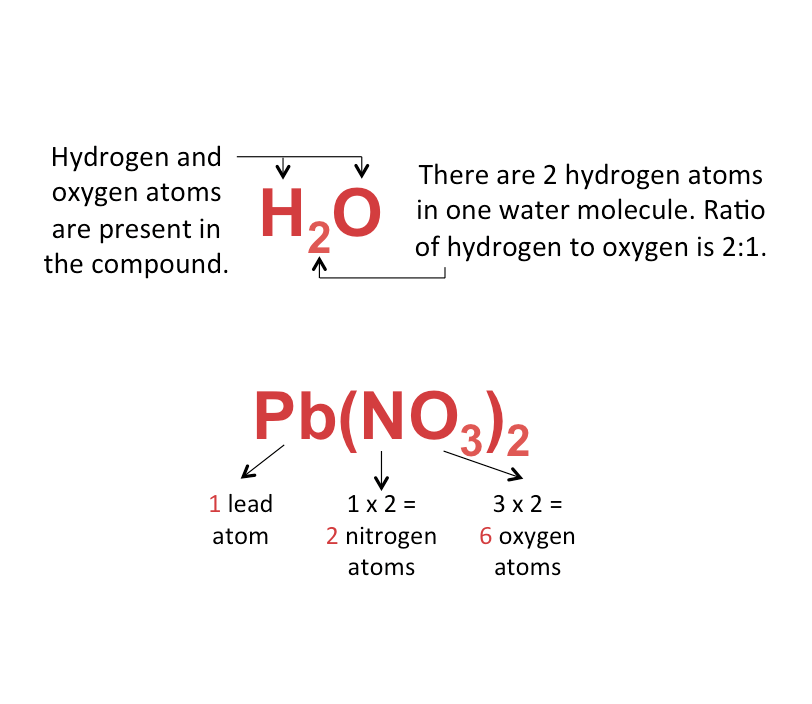
Chemical Formula of a Compound
The chemical formula of a compound is written by putting together the chemical symbols of the elements that make up the compound.
The chemical formula shows
- the types of atoms present
- ratio of different atoms present
The examples of water and lead(II) nitrate are shown below.
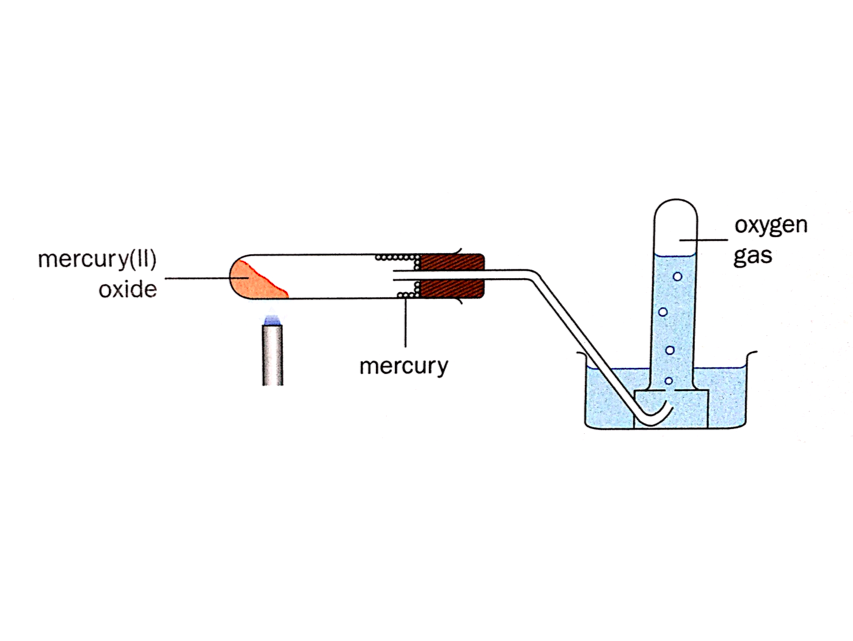
Decomposition of Compounds
Chemical processes such as thermal decomposition and electrolysis can be used to decompose compounds.
Electrolysis is the process of using electricity to break down a compound.
Thermal decomposition involves heating a compound strongly. For example, when mercury(II) oxide is heated strongly, it decomposes to give the elements mercury and oxygen.
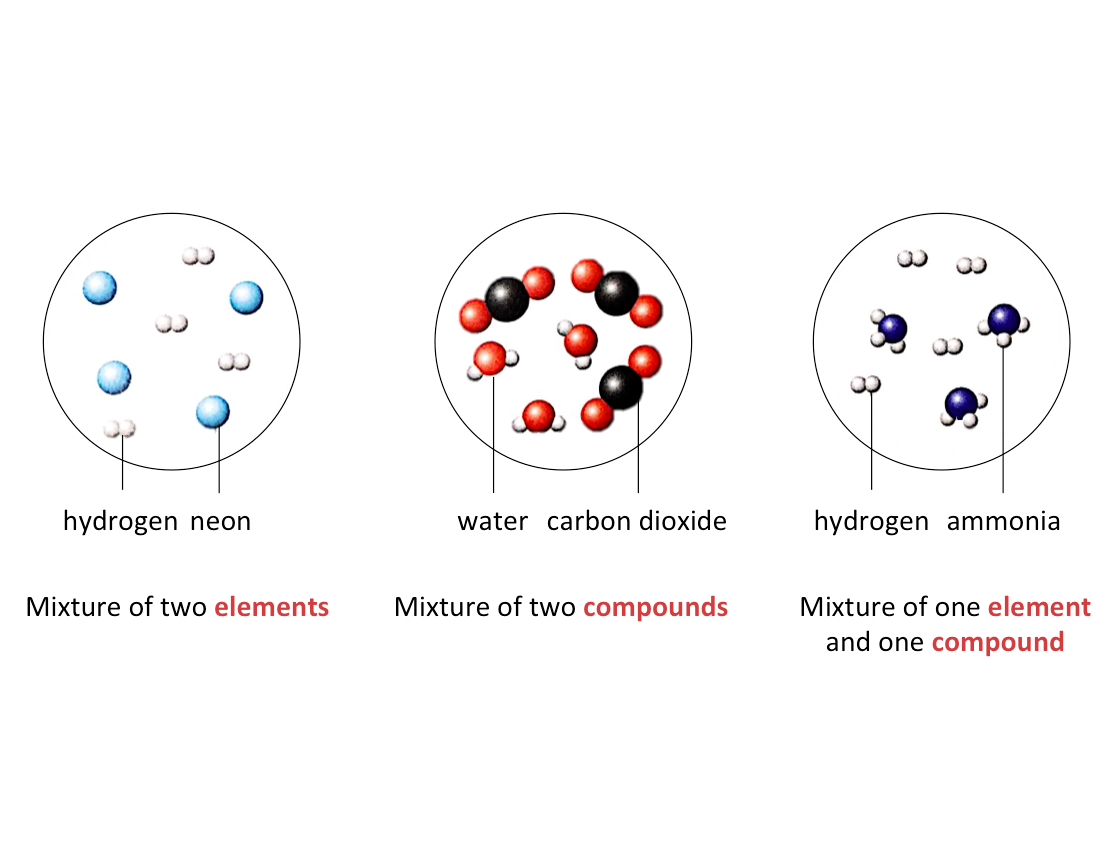
What is a mixture?
A mixture is made up of two or more substances that are not chemically combined.
- Mixtures can be made up of elements or compounds.
- Components of a mixture are not fixed and can be present in any ratio.
An example of a widely used mixture is alloys, which consist of a mixture of metals with other elements.
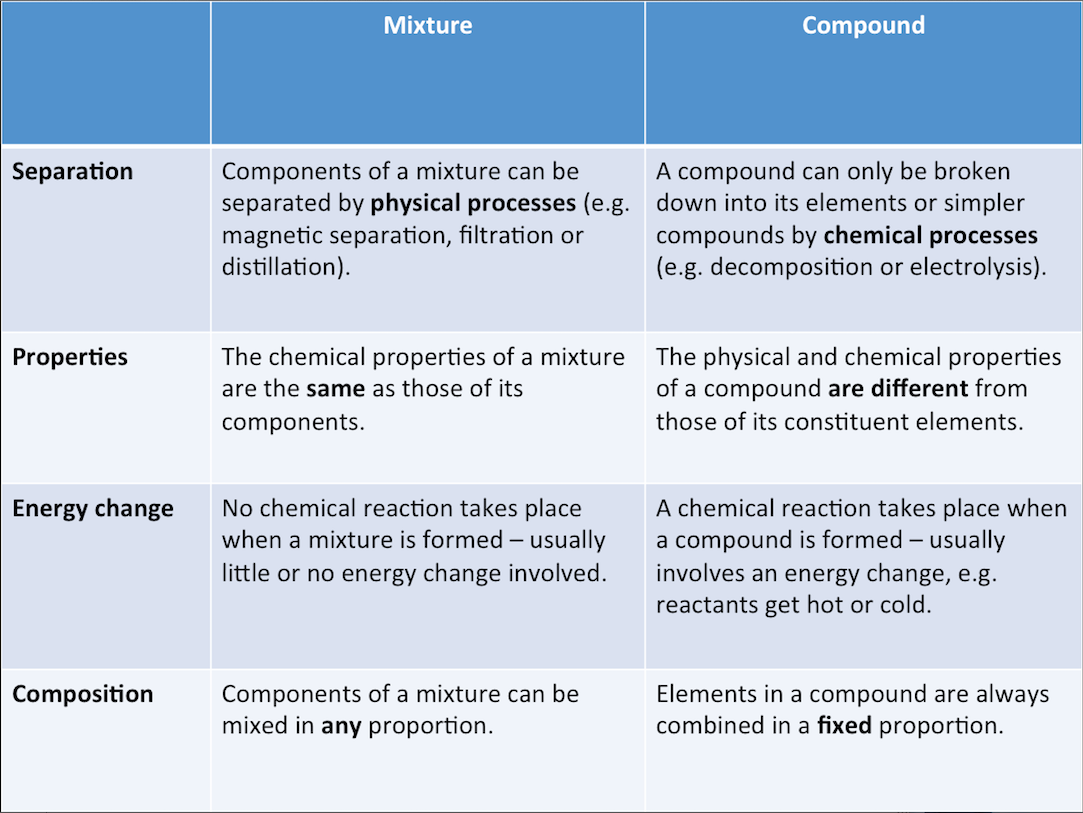
Differences Between Compounds and Mixtures
The table below shows the differences between compounds and mixtures.
Differences Between Compounds and Mixtures (Video)
To better understand the differences between compounds and mixtures, watch the video below.
Solute, Solvent, Solution and concentration
It is important to distinguish between three closely related terms solute, solvent, and solution. You are required to know the definitions for each of these term as well as the concentration of the solution.
Solute
Solute is the substance that dissolves to form a solution, for eg salt.
Solvent
Solvent is the substance in which a solute dissolves
Solution
Solution is a homogeneous mixture of one or more solutes dissolved in a solvent.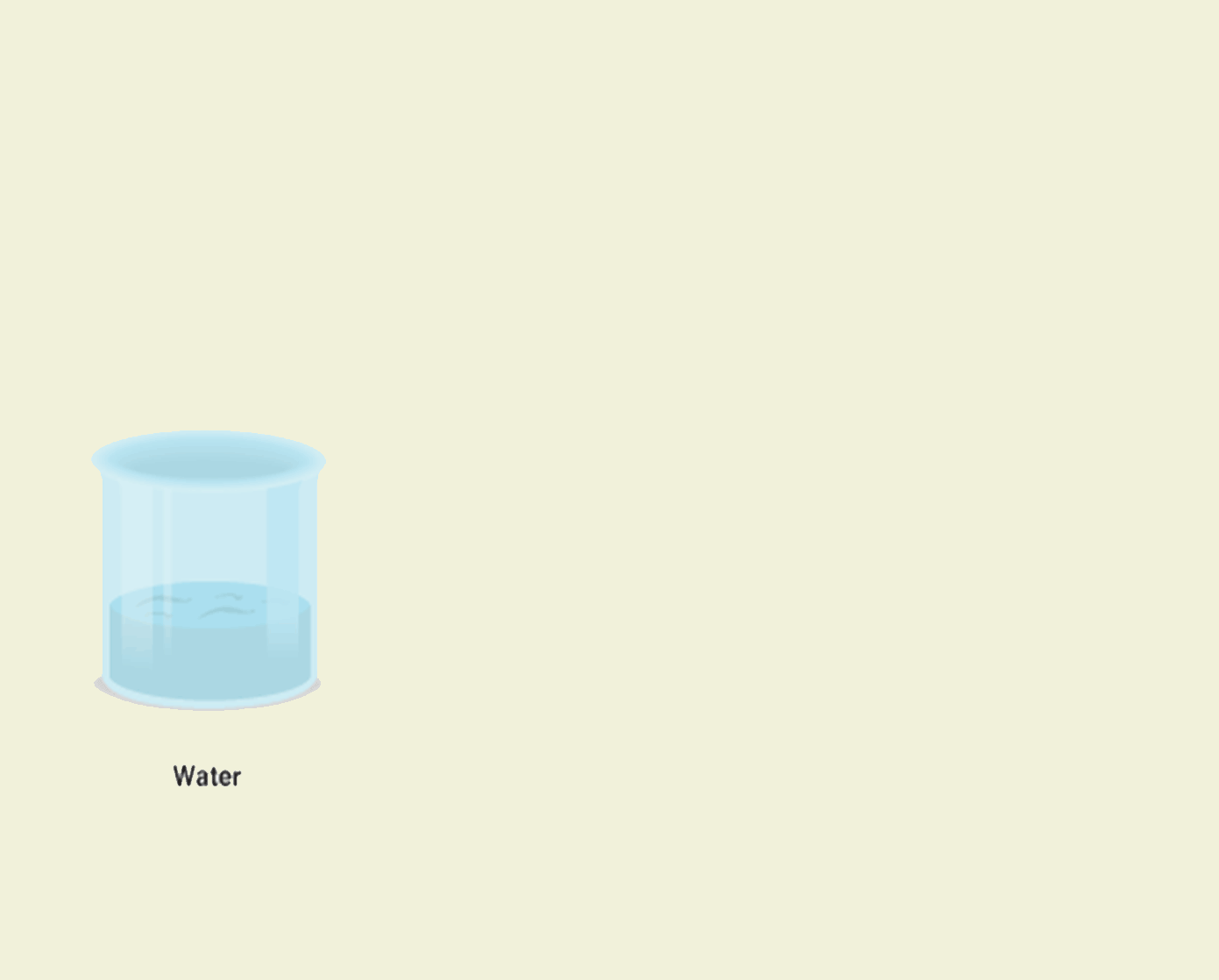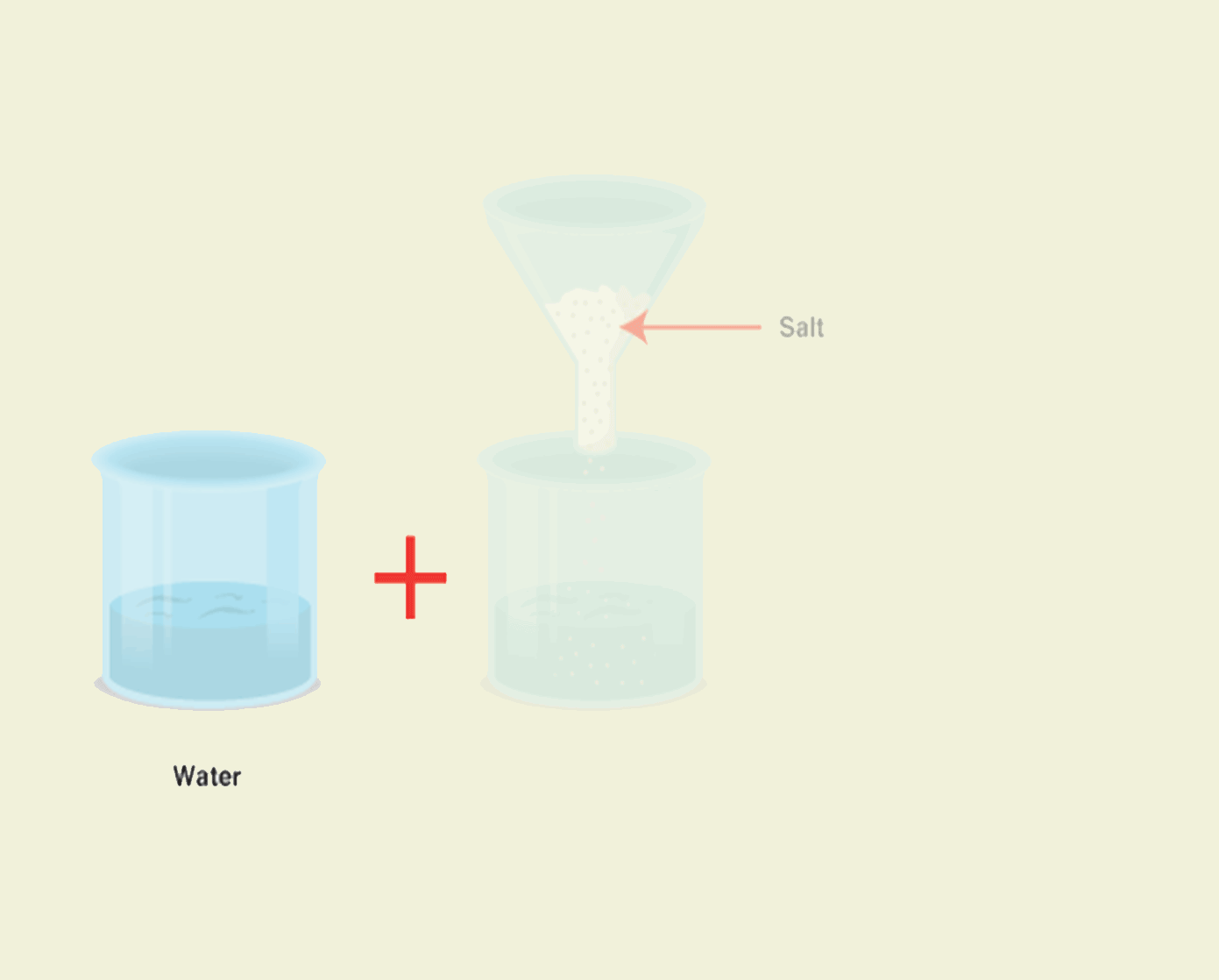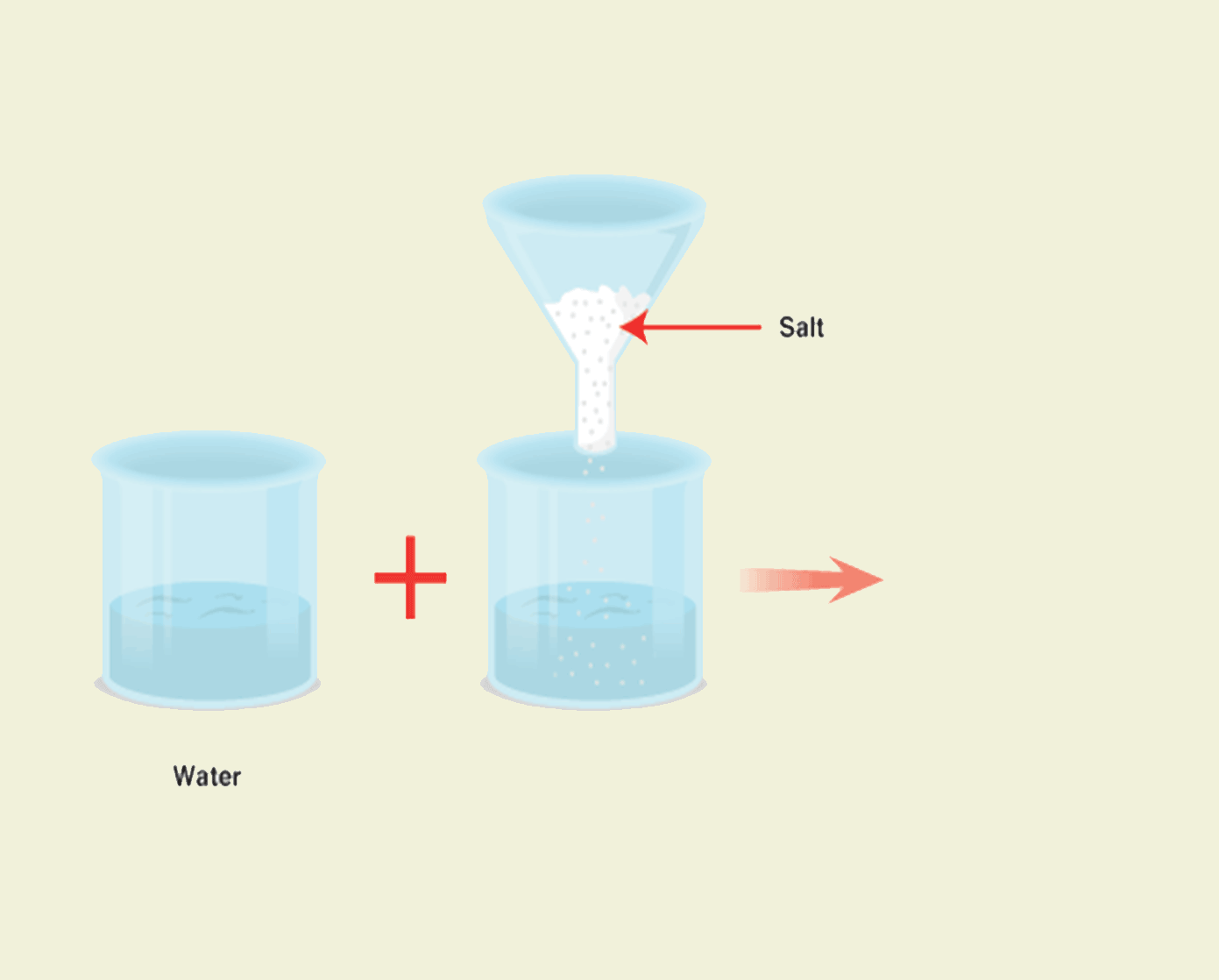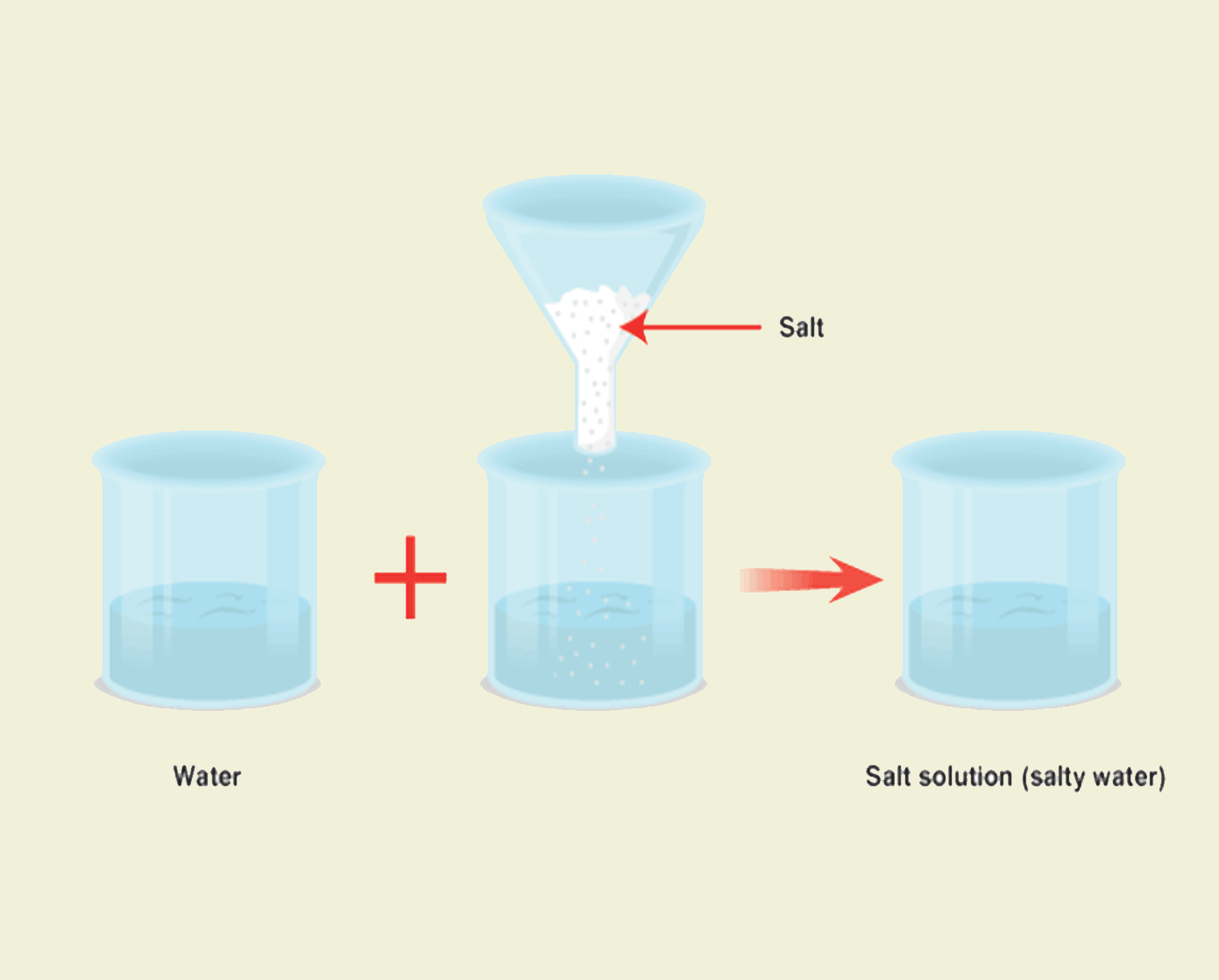
Concentration
Concentration is the ratio of the amount of solute to the amount of solvent or solution.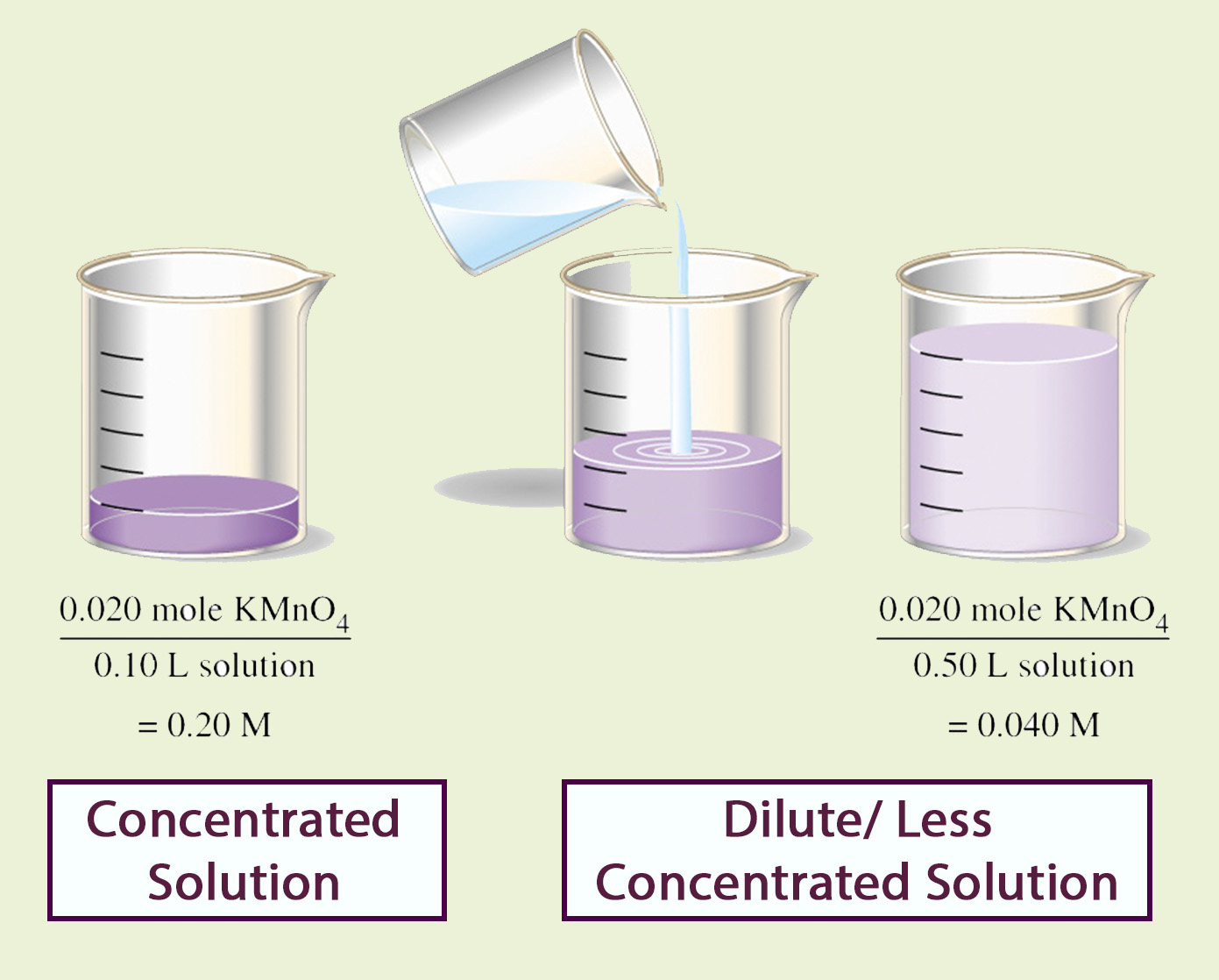
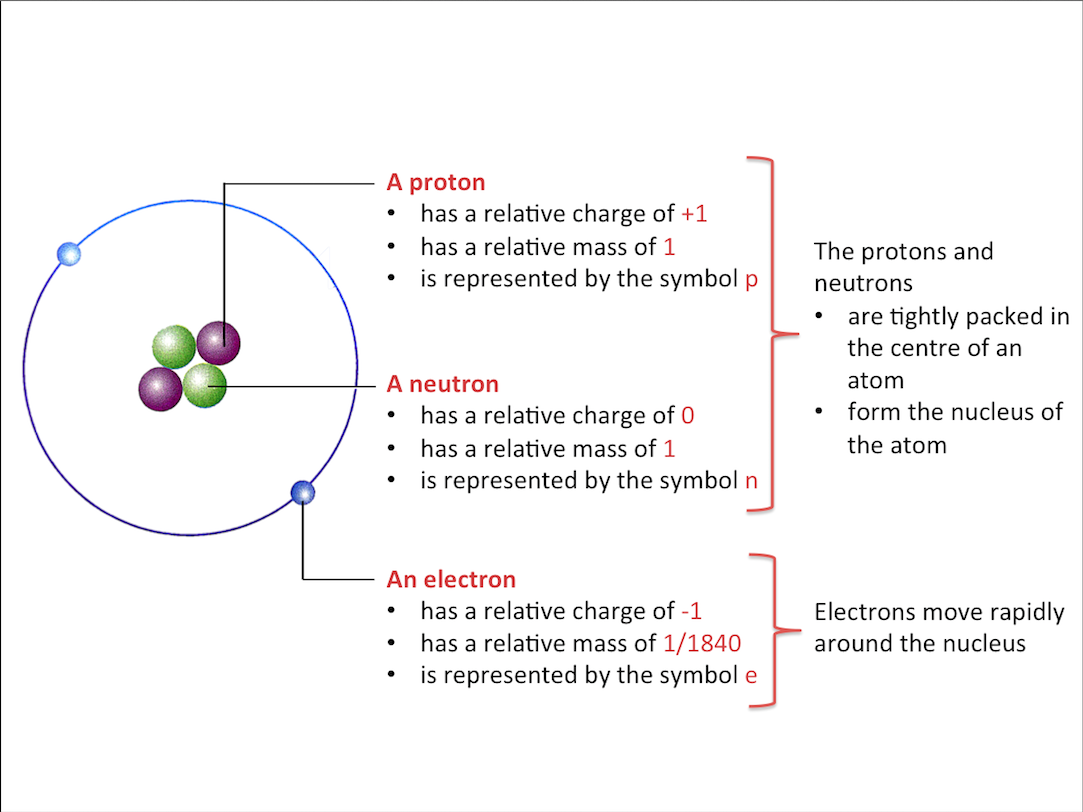
What are atoms made of?
Atoms are made up of protons,neutrons and electrons. These smaller particles are called subatomic particles.
The Atom
The number of electrons in an atom is the same as the number of protons. Thus,
- The negative charges cancel out the positive charges
- An atom is electrically neutral
Proton Number
The proton number of an atom refers to the number of protons in the atom.
Proton number is also called the atomic number, represented by the symbol Z.
Since atoms are electrically neutral, proton number = number of electrons in the atom.
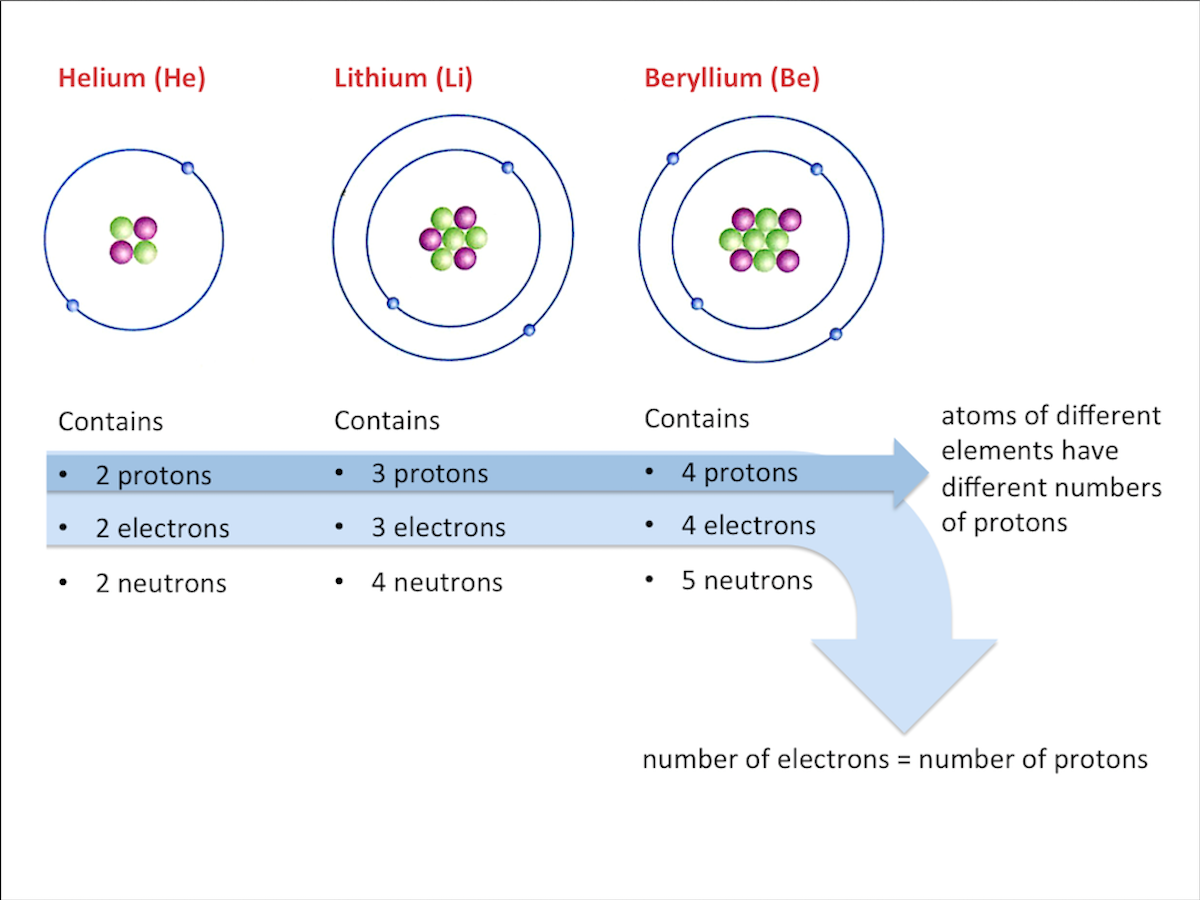
For example, the proton number of nitrogen is 7. This means that the nitrogen atom has seven protons and seven electrons.
Each element has a unique proton number. No two elements have the same proton number.
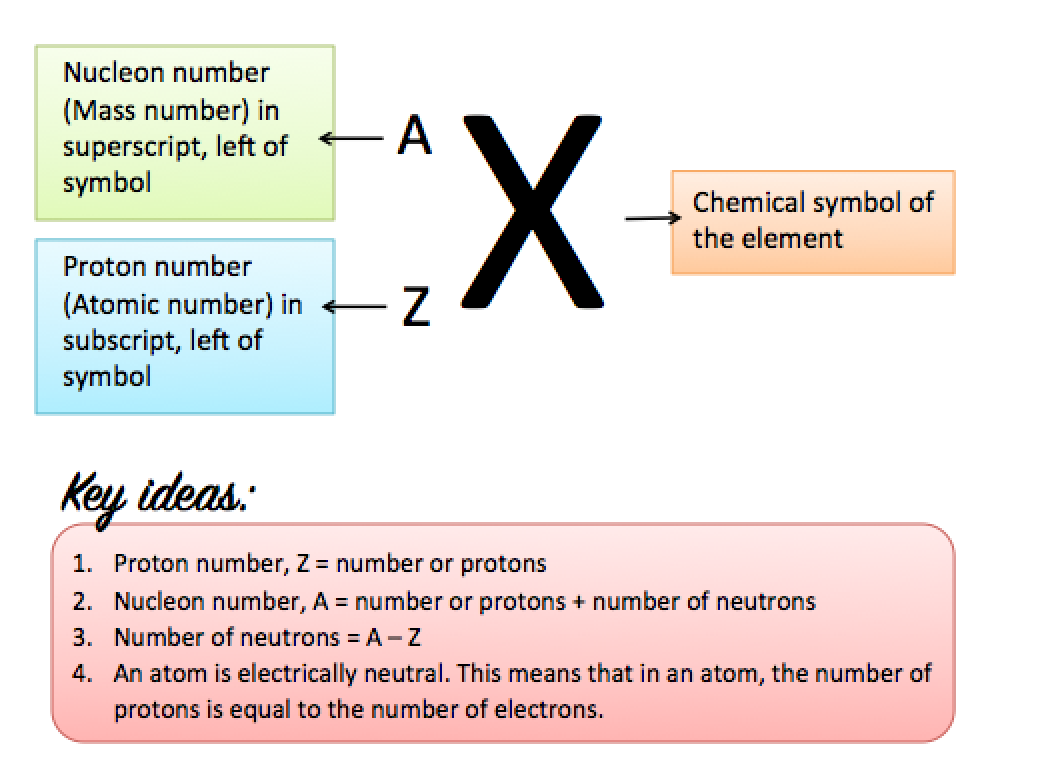
Nucleon Number
The nucleon number of an atom is the total number of protons and neutrons in the atom. It is also called the mass number, as the mass of an atom depends on the number of protons and neutrons in its nucleus. The mass of electrons in the atom is said to be negligible.
The nucleon number is represented by the symbol A.
Nucleon number (A) = mass number = number of protons + number of neutrons
Representing the Proton and Nucleon Numbers
The nucleon and proton numbers can be included when representing an element in symbols.
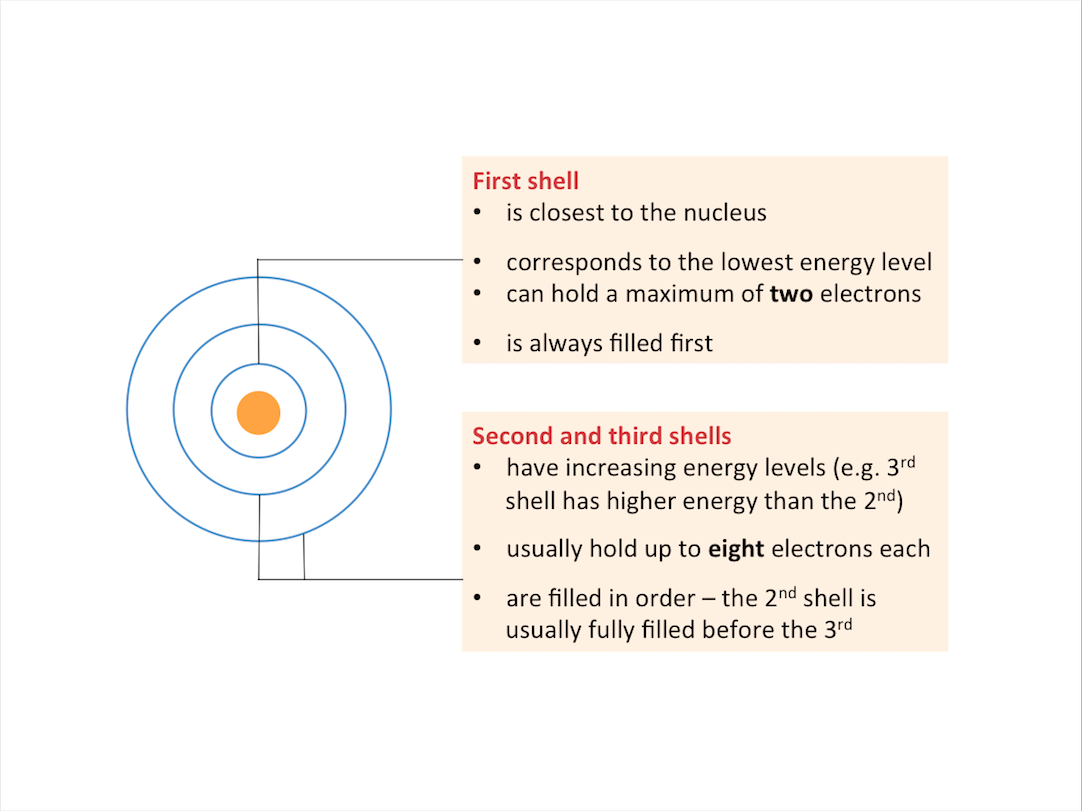
How are electrons arranged in an atom?
The electrons in an atom move around the nucleus in regions known as electron shells.
Each electron shell:
- Corresponds to a specific energy level, and
- Can only hold a certain number of electrons
Electronic Configuration
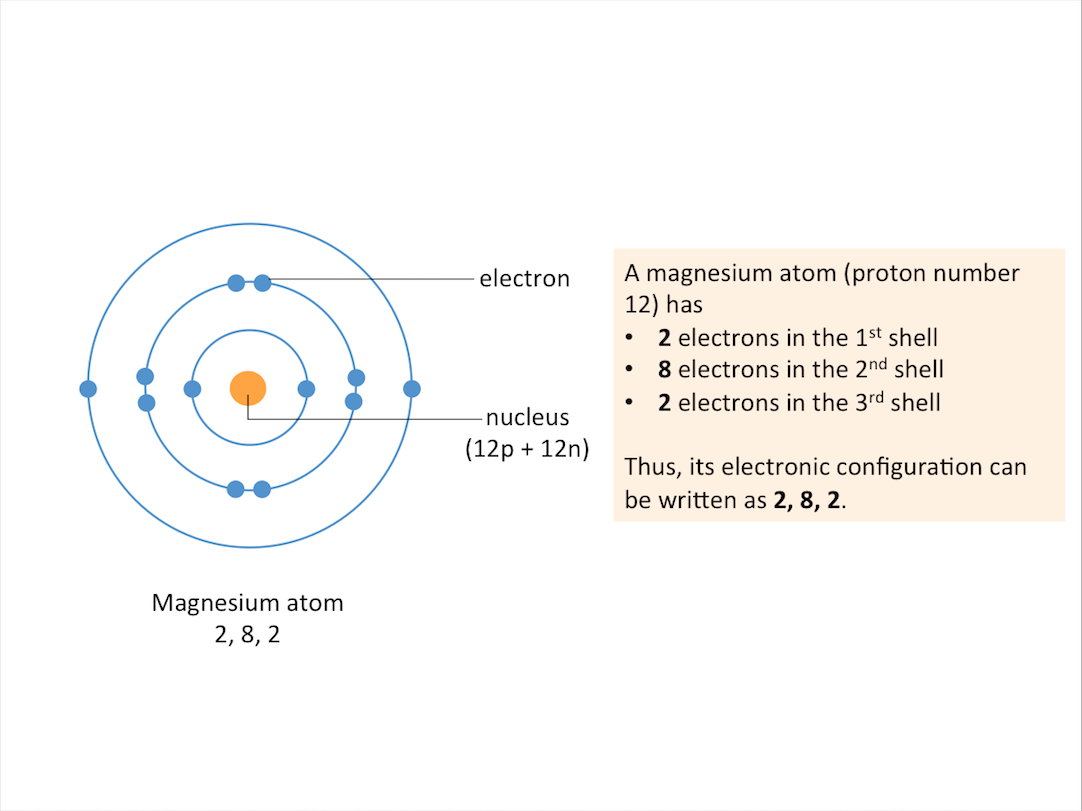
The arrangement of electrons in an atom can be represented using electronic structure or electronic configuration. The electronic structure of a magnesium atom is shown in the figure below.
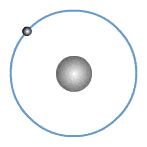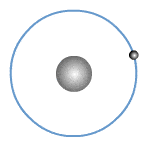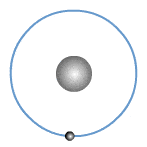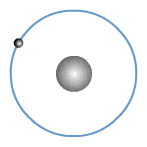
What are valence electrons?
The valence shell or outer shell of an atom refers to the shell that is furthest away from the nucleus of the atom. The electrons in the outer shell of an atom are known as valence electrons or outer electrons.
The outer electronic structure of an atom shows only the electrons in the outer shell. An example is shown for Sodium below.
In fact, all Group 1 elements have the same valency of 1, hence they have the same outer electronic structure as shown.
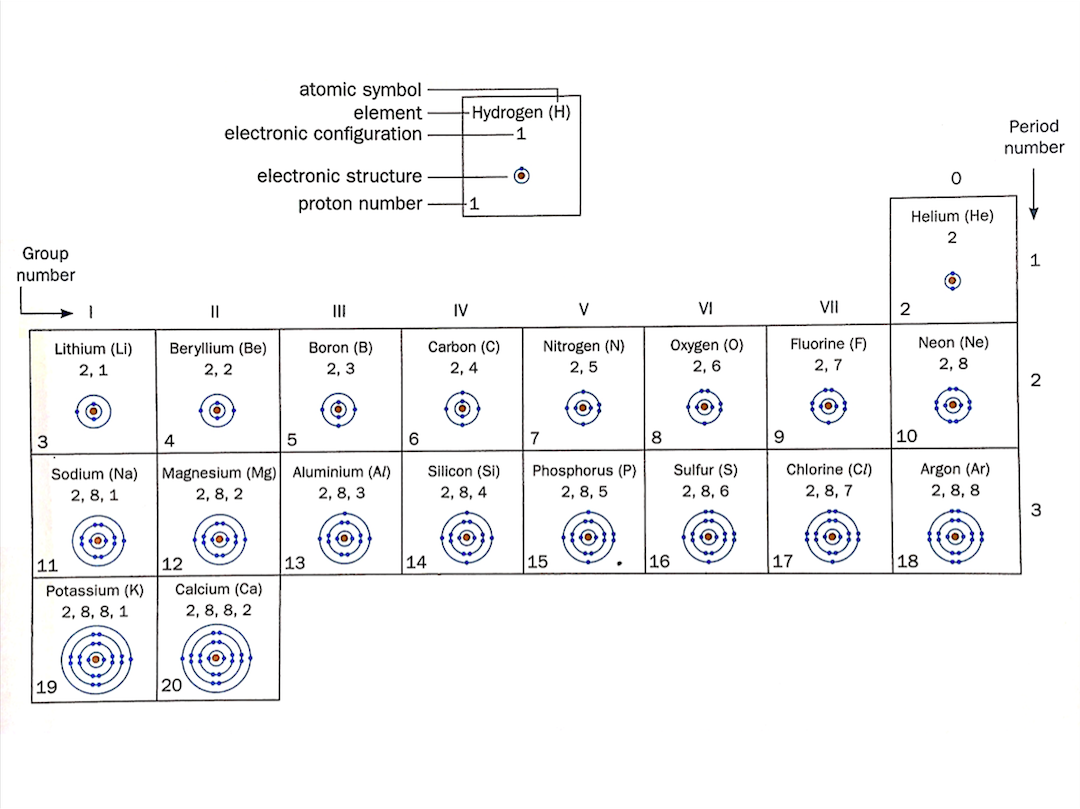
[Supplementary] Electronic Structures of Some Elements
The electronic structures of the first 20 elements in the periodic table are shown below.
In the periodic table,
- Elements are arranged in order of increasing proton number
- The (horizontal) rows of elements are called periods
- The (vertical) columns of elements are called groups
Elements in the same group have the same number of valence electrons and elements in the same period have the same number of electron shells.
How are electrons arranged in an atom?
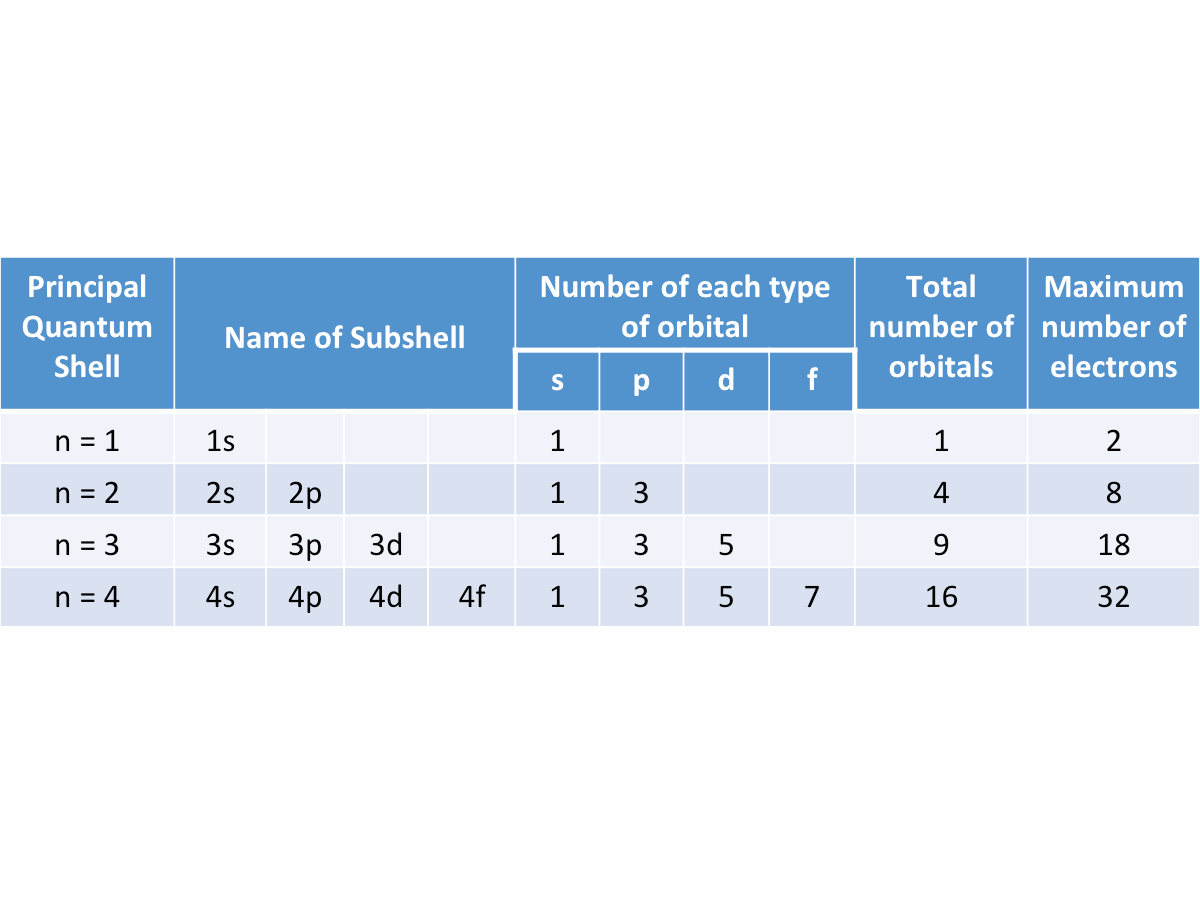
Principal Quantum Shell:
- A shell is a group of orbitals that are about the same distance from the nucleus.
- n = 1 is the shell closest to the nucleus.
Subshell:
- A subshell is a group of orbitals with the same energy level (degenerate), but with different orientation in space.
Orbital:
- An orbital is a region of space where there is a high possibility that electrons on an atom can be found, with no sharp boundary surface to the diffused electron cloud.
- The s, p ,d and f orbitals differ in shape and orientation in space.
Electron
- Each electron spins on its own axis.
- The maximum number of electrons that can be fitted into an orbital = 2 one spins in one direction, the other spins in the opposite direction.
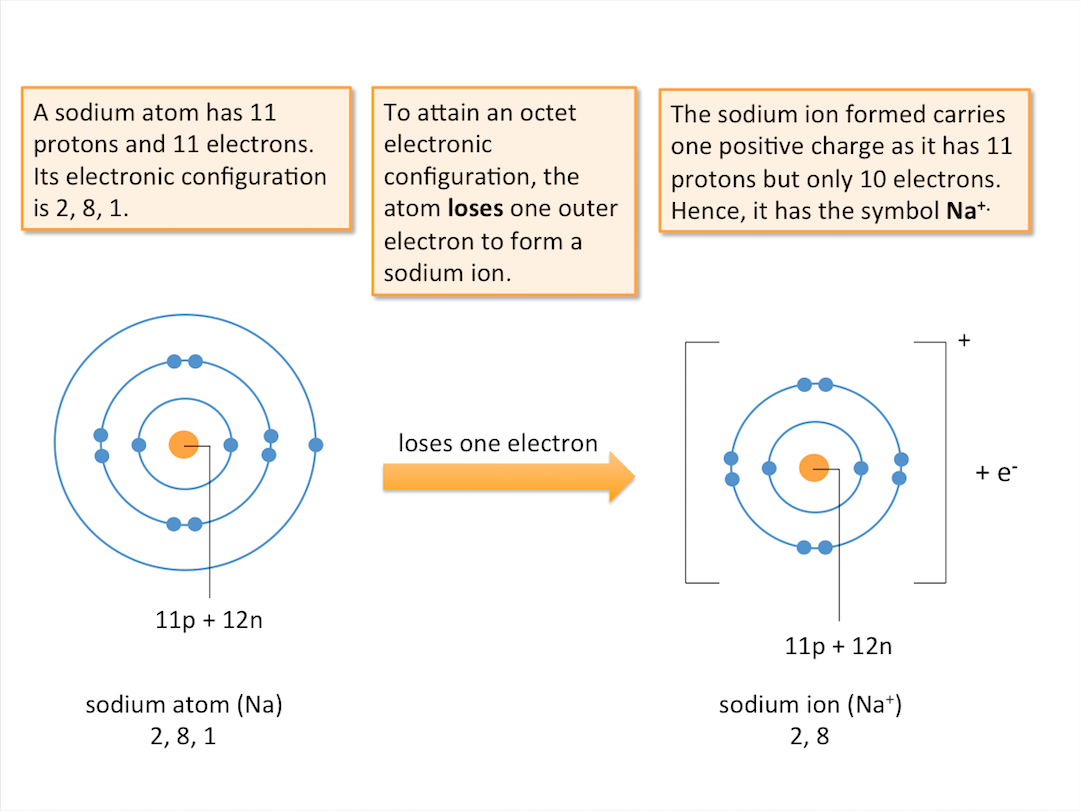
Formation of Positive Ions (Cations)
Atoms of metals tend to lose electrons to form cations, attaining the electronic configuration of a noble gas. When an atom loses electrons, it has more protons than electrons. A positive ion or cation is formed. The charge on a cation corresponds to the number of electrons the atom loses.
Positive ions or cations are formed when atoms lose electrons.
Formation of Positive Ions (Example)
The diagram below shows the formation of a sodium ion.
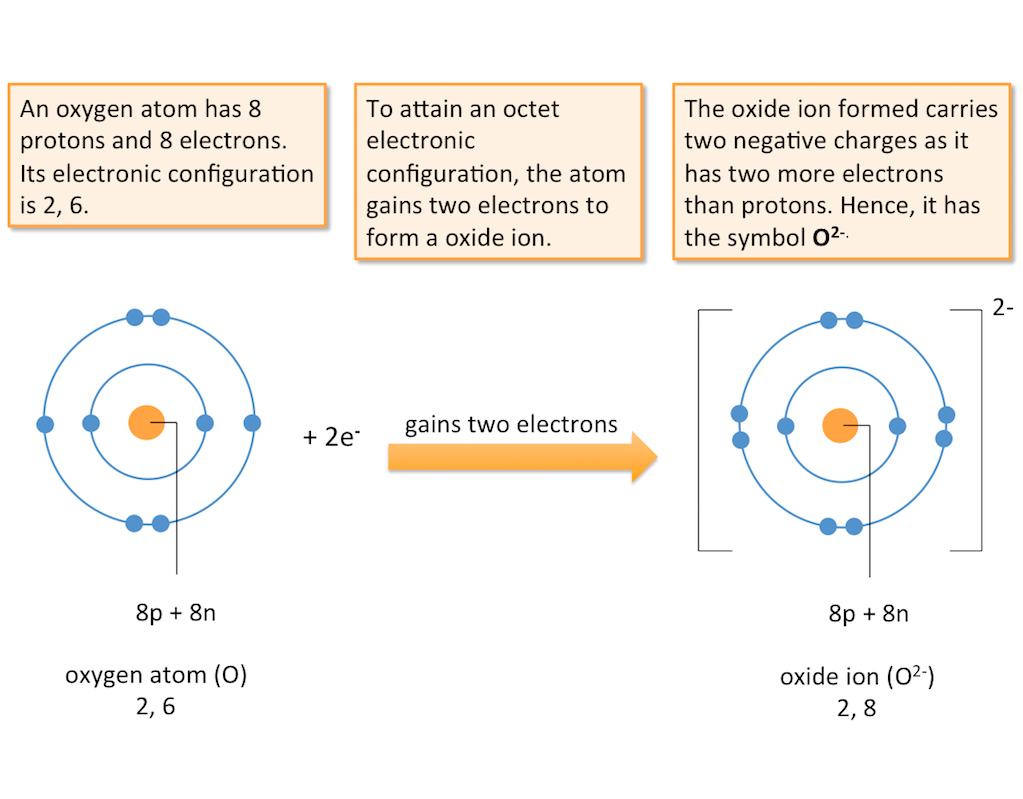
Formation of Negative Ions (Anions)
Atoms of non-metals usually have more than four outer electrons. Hence, they tend to gain electrons to form anions.
When an atom gains electrons, it has more electrons than protons. A negative ion or anion is formed. The charge on an anion corresponds to the number of electrons the atom gains.
Negative ions or anions are formed when atoms gain electrons.
Formation of Negative Ions (Example)
The diagram below shows the formation of an oxide ion.
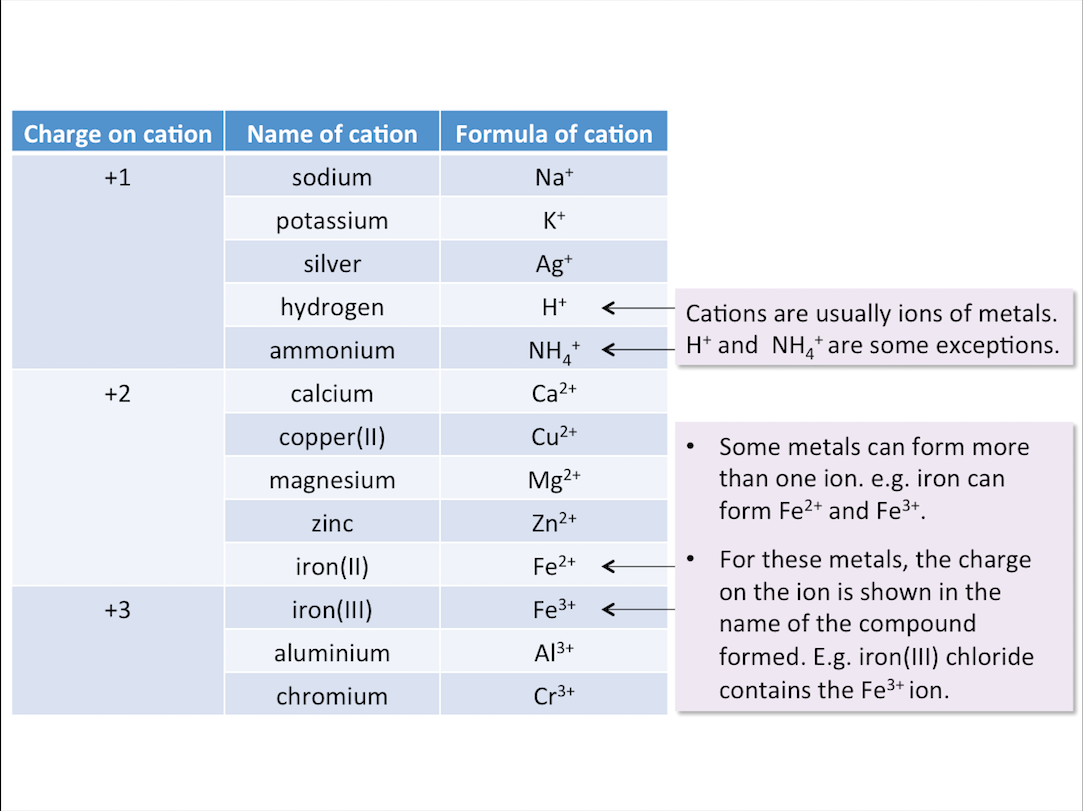
Common Cations and Their Charges
The table below shows the names and formulae of some cations.
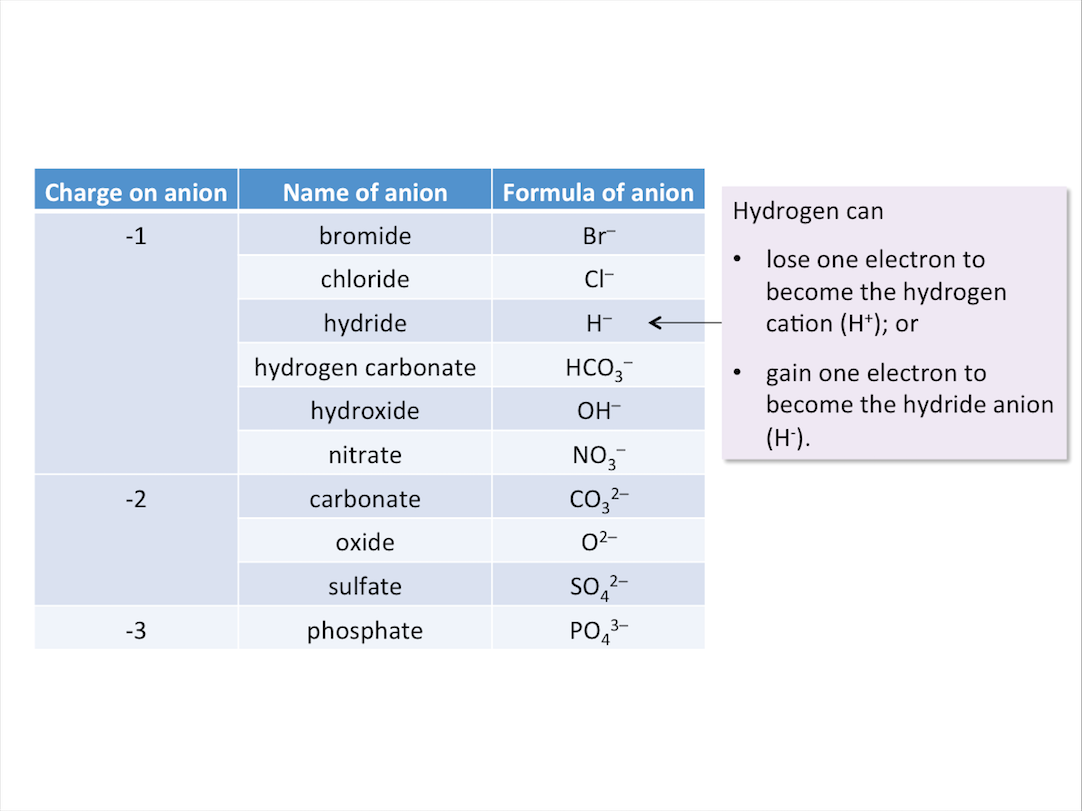
Common Anions and Their Charges
The table below shows the names and formulae of some anions.
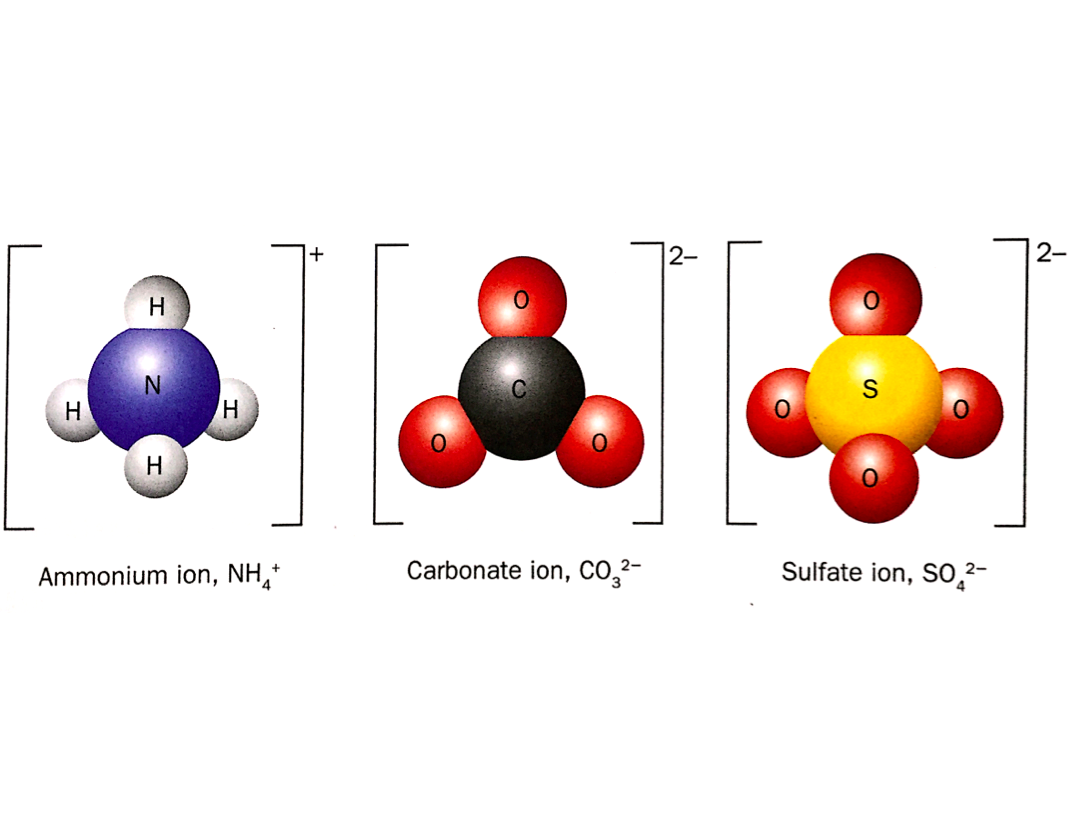
Polyatomic Ions
The ions below are polyatomic ions. They are ions composed of two or more covalently bonded atoms.
Ionic Bonds
- Ionic bonds are the strong electrostatic forces of attraction between positive and negative ions.
- It involves the transfer of electrons from one atom to another atom.
- It occurs between metals and non-metals
- Metals <
Ionic bonds are the strong electrostatic forces of attractive between positive and negative ions.
[Supplementary] Formation of an Ionic Bond
The formation of an ionic bond can be shown by a dot and cross diagram.
In a dot and cross diagram,
- dots represent the electrons of one atom
- crosses represent the electrons of another atom
The figure below shows the steps involved in the formation of sodium chloride.
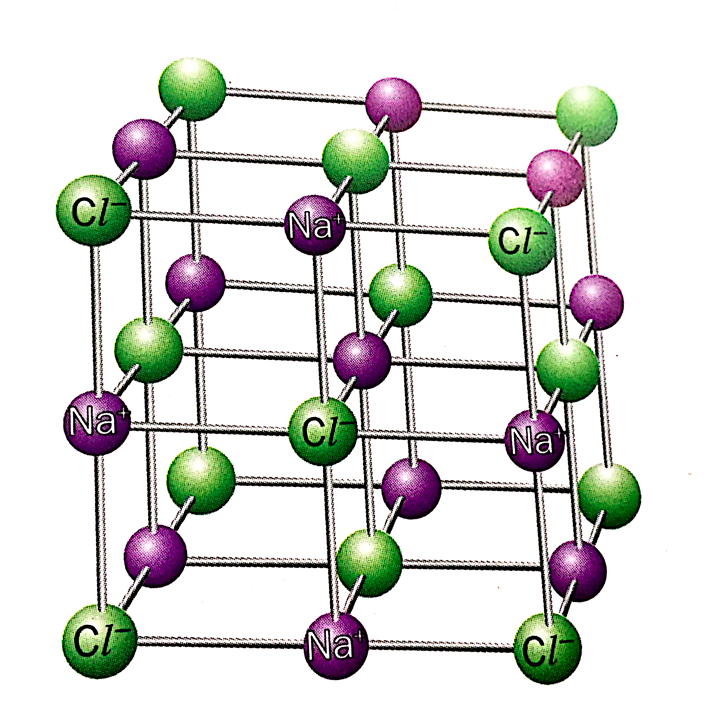
Giant Lattice Structures of Ionic Compounds
Ionic compounds form giant lattice structures.
Sodium chloride is a typical example of an ionic compound.
- The sodium and chloride ions are arranged in a giant lattice structure.
- A giant lattice structure is a 3D network of ions.
- The ions are held in place by ionic bonds.
- The ions are packed in a regular, repeating pattern.
- The giant lattice structure is held together tightly as the oppositely charged ions attract one another strongly.
How can we deduce the formula of sodium chloride from its lattice structure?
In the lattice structure of sodium chloride,
- Each Na+ ion is surrounded by six Cl- ions
- Each Cl- ion is surrounded by six Na+ ions
- The overall ratio of Na+ to Cl- ions is 1:1
Hence, NaCl is the formula unit of sodium chloride.
[Supplementary] Physical Properties of Ionic Compounds
1. High melting points and boiling points
In an ionic compound, the forces of attraction between the oppositely charged ions are very strong and require a large amount of heat energy to be overcome. Due to their high melting and boiling points, most ionic compounds are solids are room temperature and pressure. Ionic compounds are non-volatile.
2. Ionic compounds are usually soluble in water and insoluble in organic solvents.
Water molecules are attracted to ions, weakening the electrostatic forces of attraction between the ions. As a result, the ions are pulled from the lattice structure and the compound dissolves to form an aqueous solution. In organic solvents, there is no water present, thus ionic compounds are insoluble as the ions remain tightly held in the lattice structure.
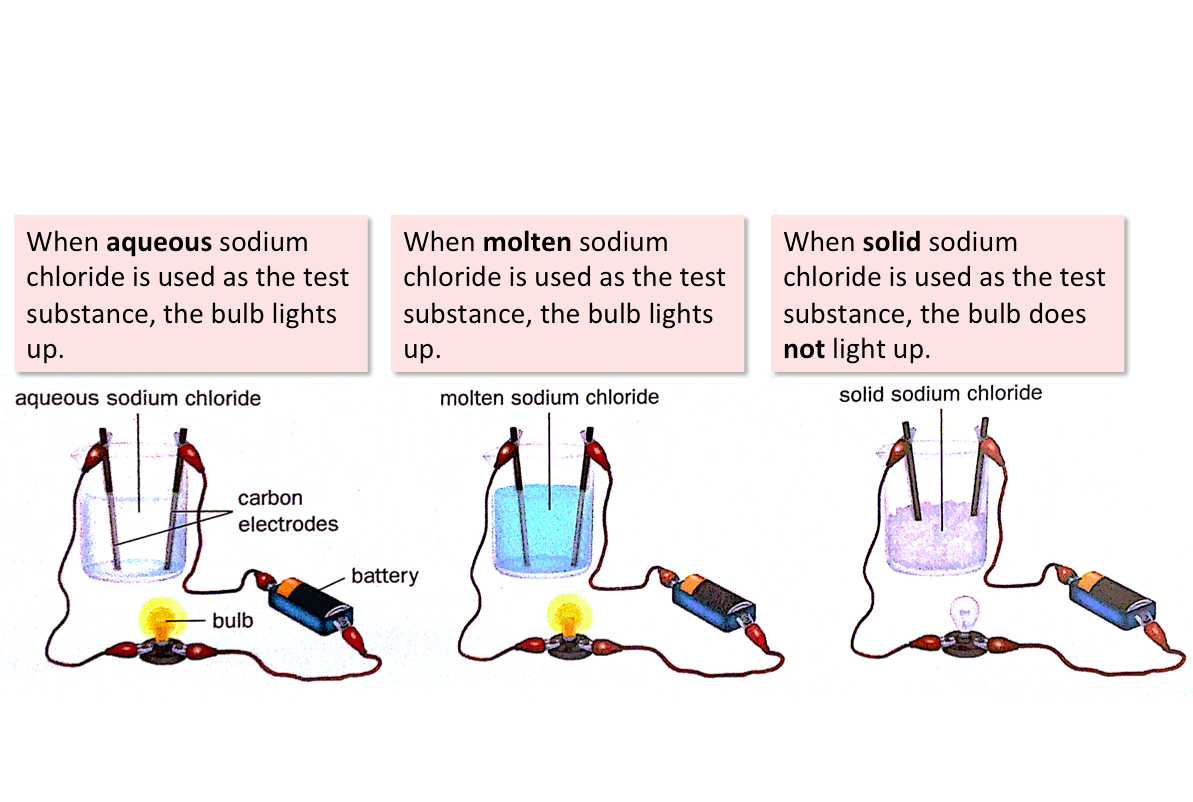
3. Ionic compounds conduct electricity when molten or in aqueous solution (dissolved in water).
There must be free-moving ions or electrons in
Covalent Bond: Sharing Electrons
Covalent bonding is the sharing of electrons equally between atoms of non-metals in order to attain the electronic structure of a noble gas.
The number of covalent bonds between atoms depends on the number of pairs of shared electrons.
Single bond: 1 pair of shared electrons (e.g. H-H)
Double bond: 2 pairs of shared electrons (e.g. O=O)
Triple bond: 3 pairs of shared electrons (e.g. N?N)
Formation of a Covalent Bond
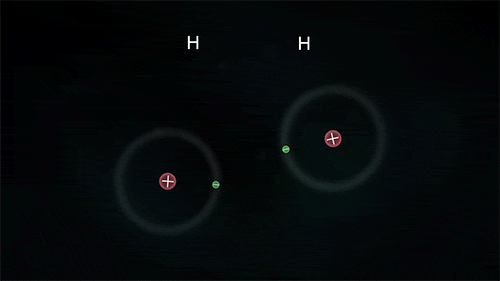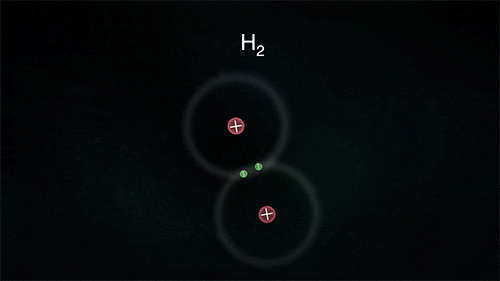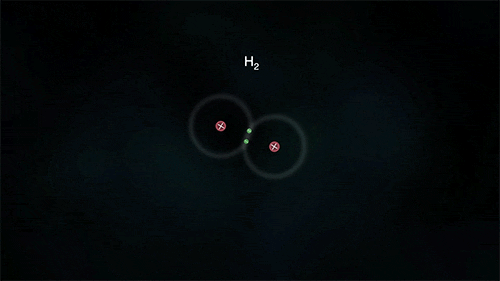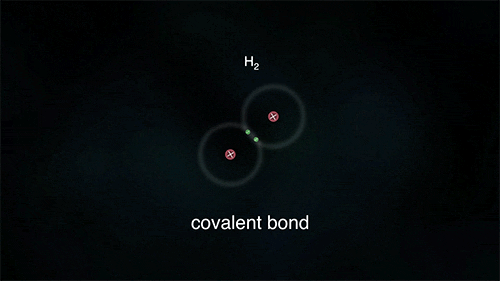
Arrangement of Electrons in Molecules (Hydrogen)
Hydrogen
The diagram below shows the formation of a hydrogen molecule.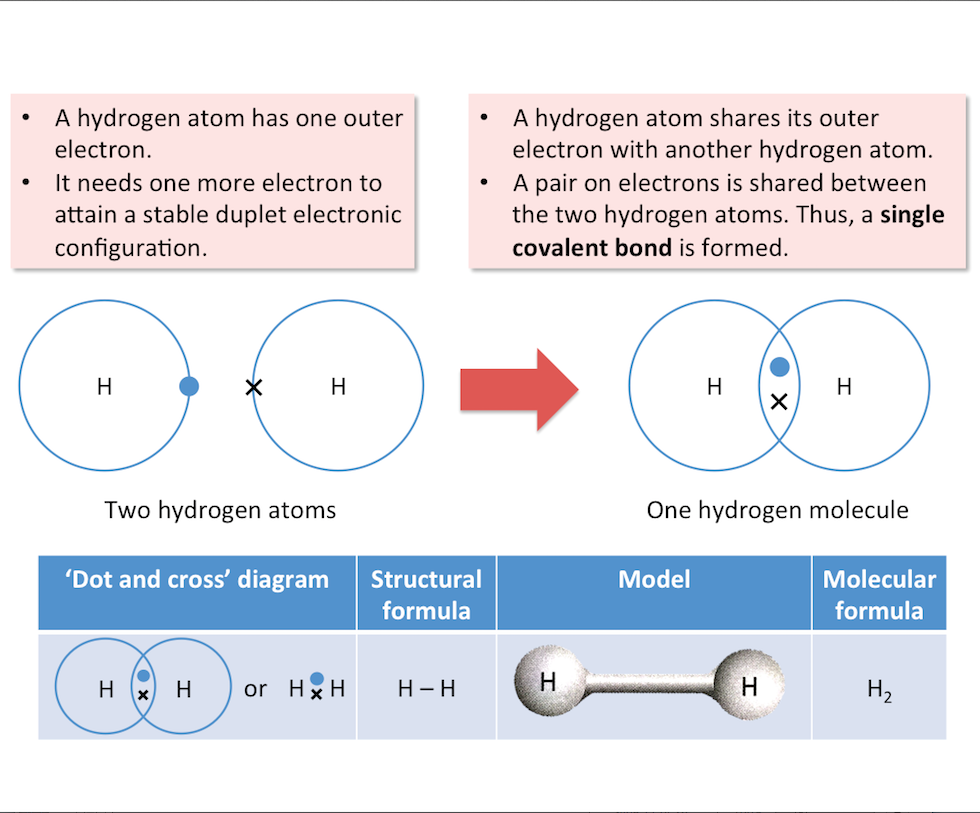
Oxygen
The diagram below shows the formation of an oxygen molecule.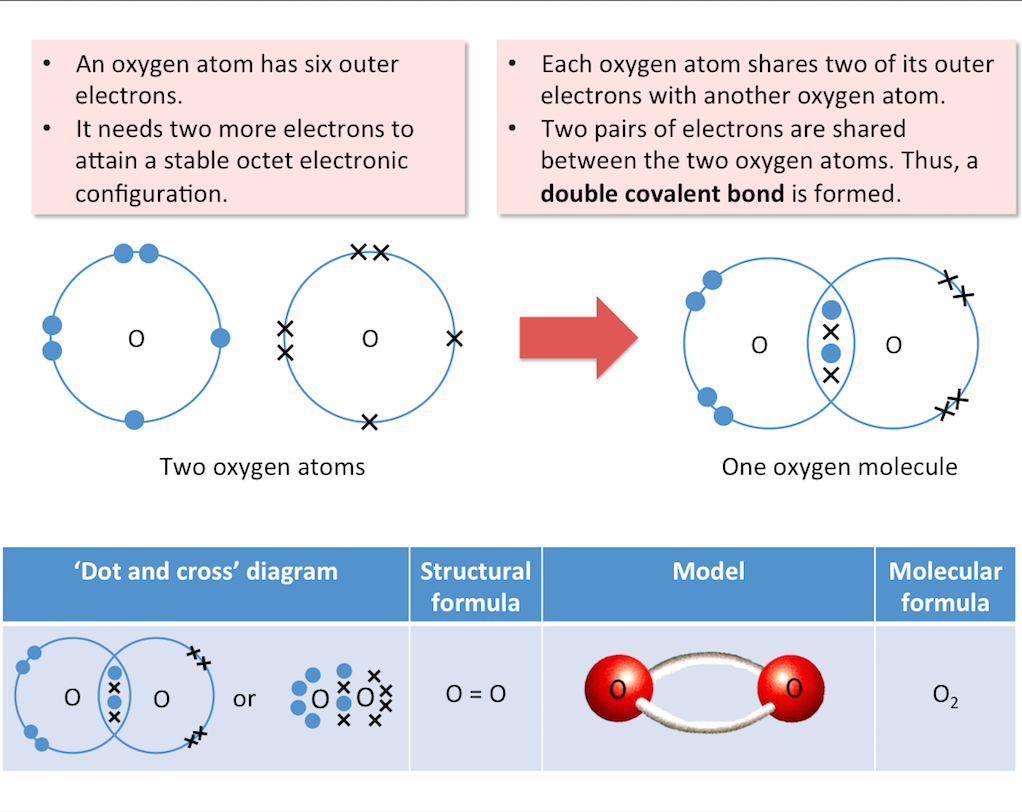
Nitrogen
The diagram below shows the formation of a nitrogen molecule.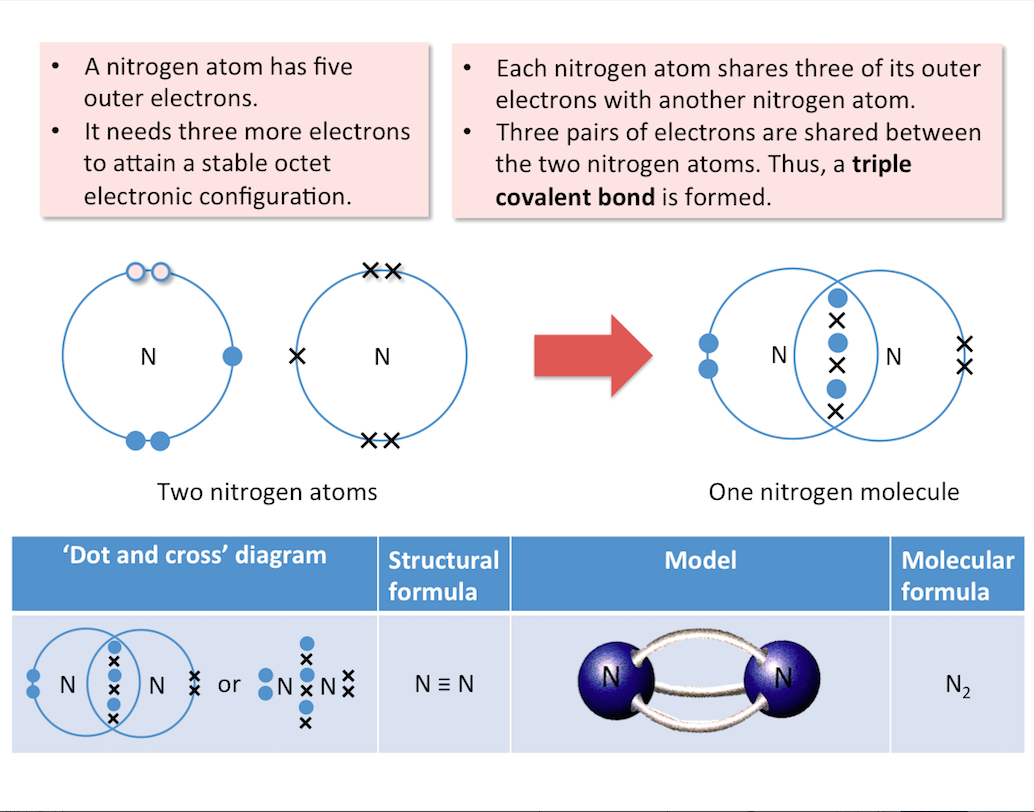
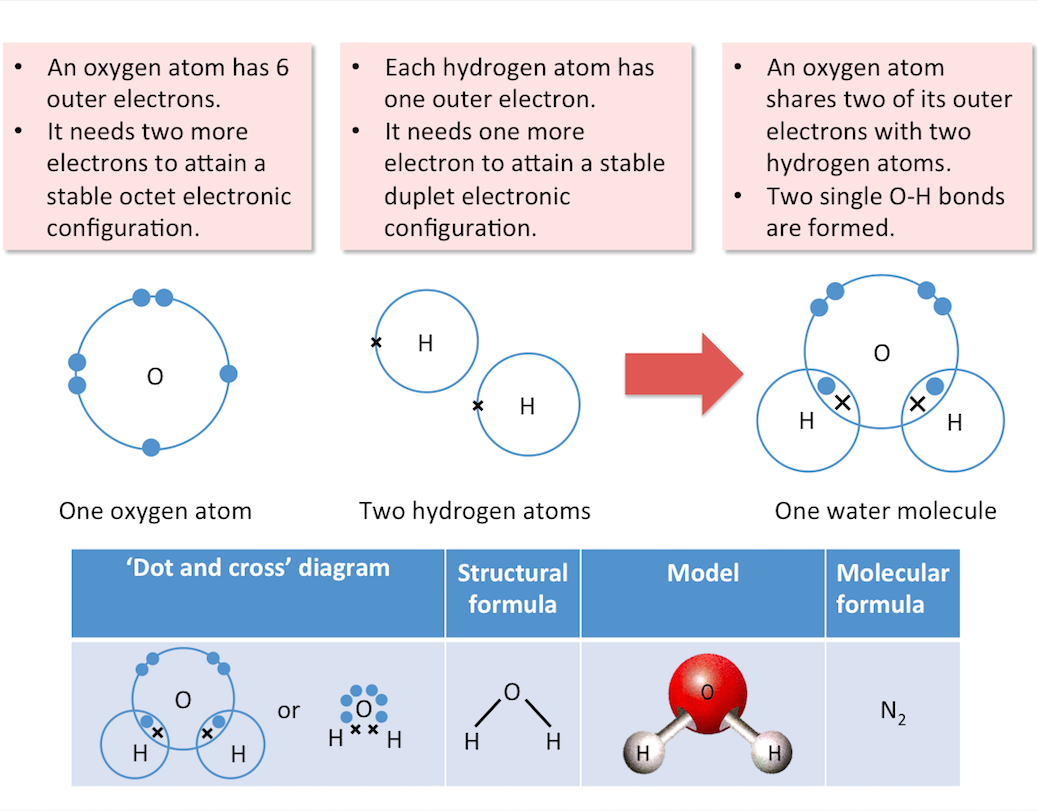
Arrangement of Electrons in Compounds
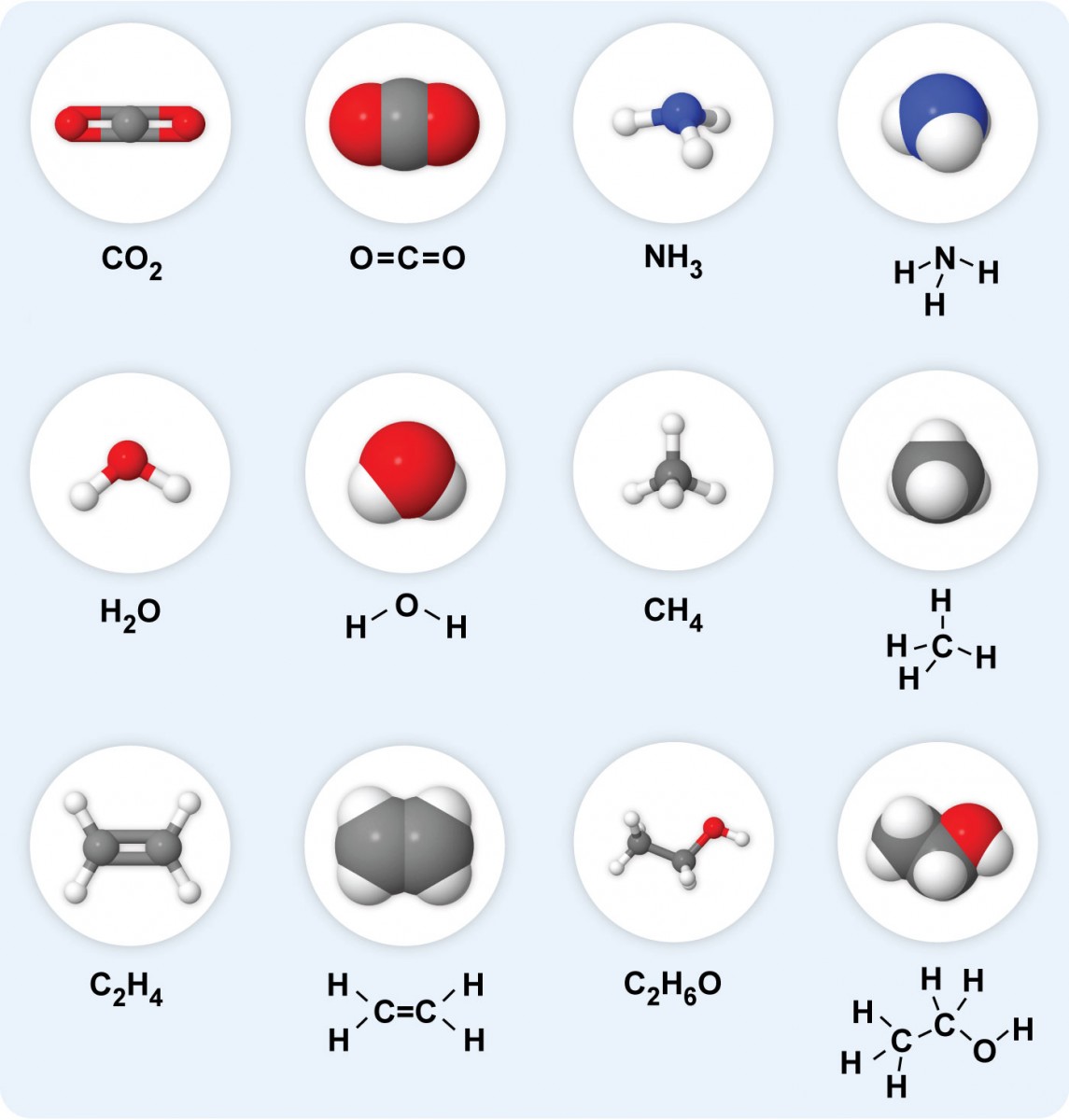
When atoms of different elements are joined together by covalent bonding, a covalent compound or molecular compound is formed.
Water, methane and carbon dioxide are examples of covalent compounds.
The diagram below shows the formation of a water molecule.
Simple Molecular Structures
Most covalent substances, such as bromine, exist as simple molecules and have simple molecular structures.
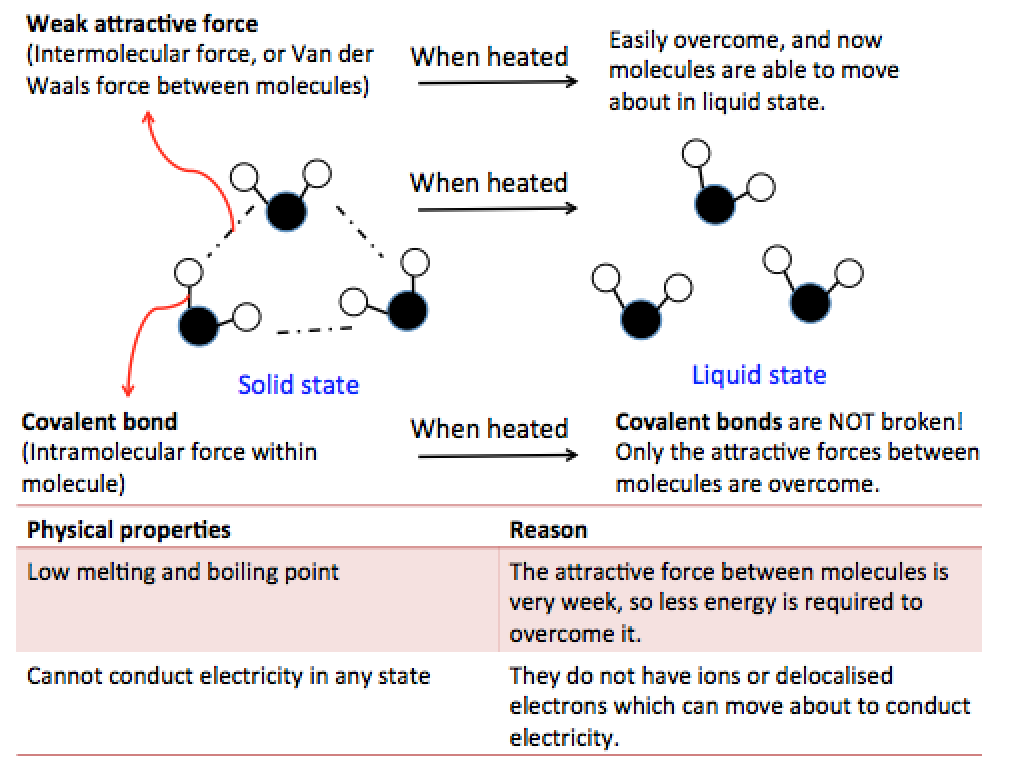
[Supplementary] Physical Properties of Simple Molecules
Most covalent substances, such as bromine, exist as simple molecules and have simple molecular structures. They have:
1. Low melting and boiling points
- Within each molecule, the atoms are held together by strong covalent bonds.
- However, between the molecules, there are only weak intermolecular forces holding them together.
- The weak intermolecular forces can be easily overcome.
Hence, simple molecules have low melting and boiling points. They are usually liquids or gases at room temperature and are volatile.
2. Insoluble in water and soluble in organic solvents.
3. Most do not conduct electricity in any state.
This is because they do not have any free-moving ions or electrons to conduct electricity.