Naming a Compound
The slides below shows some general rules for naming compounds.
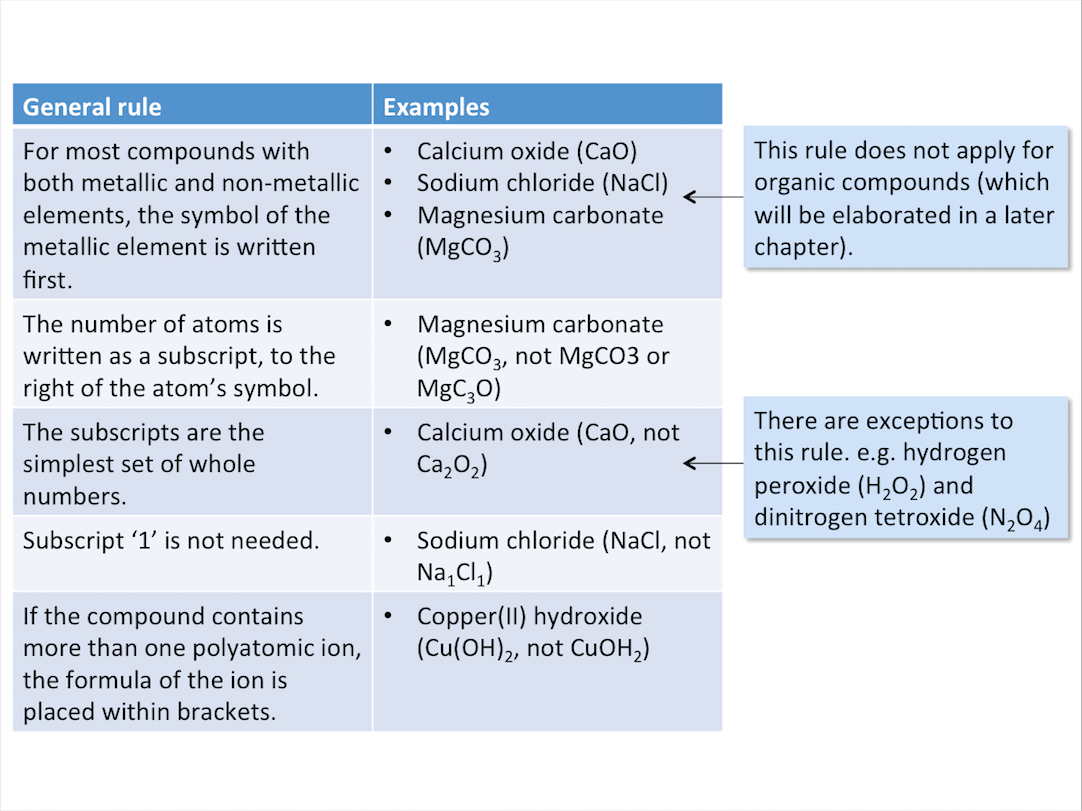
General Rules for Writing Chemical Formulae
The table below shows some general rules for writing chemical formulae of ionic compounds.
[Supplementary] Determining the Chemical Formula of an Ionic Compound
All the positive charges must be equal to all the negative charges in an ionic compound. Hence, the formula of an ionic compound can be constructed by balancing the charges on the positive ions with those on the negative ions.
Consider the ionic compound calcium chloride. The ions present are Ca2+ and Cl-. The Ca2+ion has two positive charges but the Cl- ion has only a single negative charge. Thus, there must be two Cl- ions to balance out the two positive charges. The chemical formula of calcium chloride is thus CaCl2.
To deduce the formula of an ionic compound,
1. Write down the ions with the charges, e,g, Xm+Yn-.
2. Move the values m and n diagonally (without the charges). The chemical formula is XnYm.
[Supplementary] Determining the Chemical Formula of an Ionic Compound (Example)
Below is an example of how the chemical formula of an ionic compound can be determined.
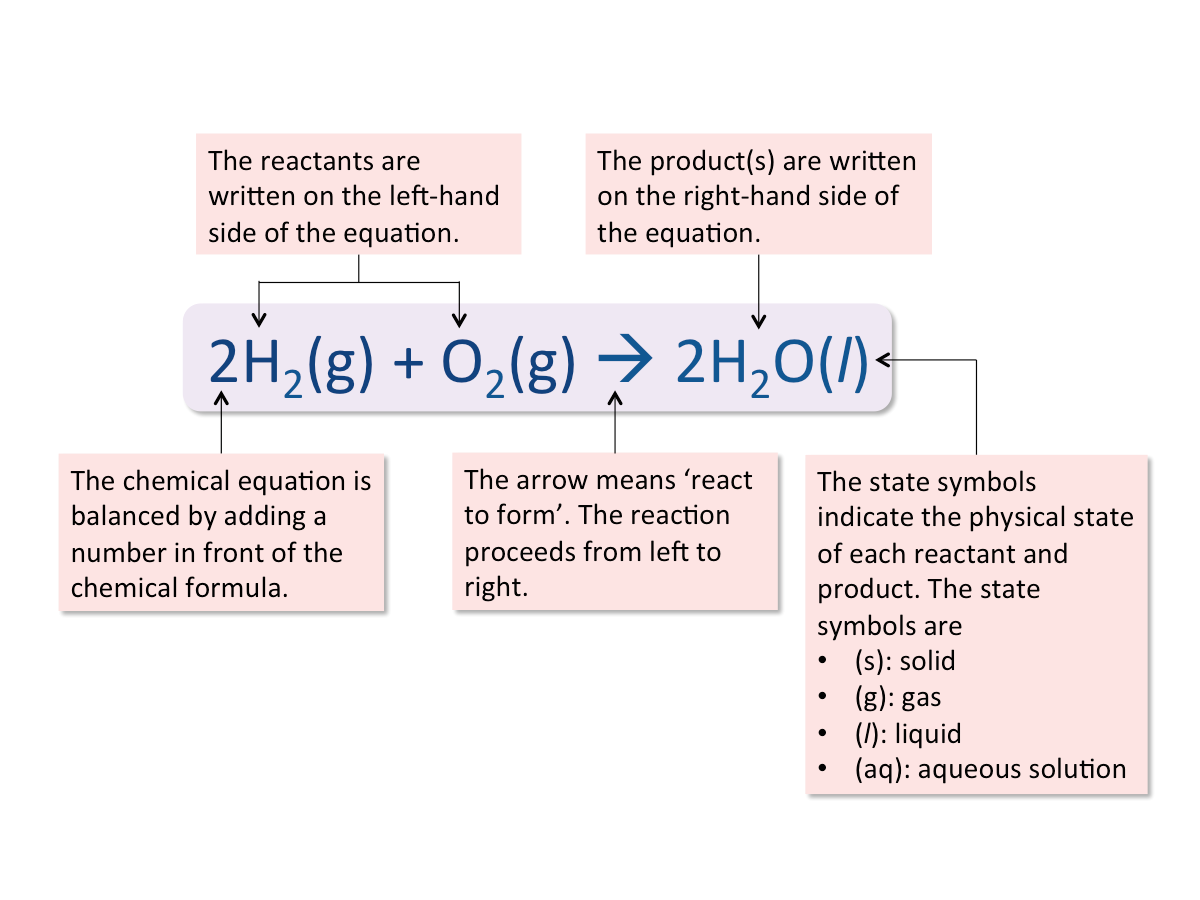
Writing Balanced Chemical Equations
A chemical equation shows what happens in a chemical reaction.
It tells us:
- Which reactants and products are involved in the reaction
- The relative amounts of reactants and products
- The physical states of the reactants and products of the reaction
A balanced chemical equation must contain an equal number of atoms of each element on both sides of the equation. A balanced chemical equation should consist of the following components:
Steps in Writing a Balanced Chemical Equation
Most covalent substances, such as bromine, exist as simple molecules and have simple molecular structures.
They have:
1. Low melting and boiling points
- Within each molecule, the atoms are held together by strong covalent bonds.
- However, between the molecules, there are only weak intermolecular forces holding them together.
- The weak intermolecular forces can be easily overcome. Hence, simple molecules have low melting and boiling points. They are usually liquids or gases at room temperature and are volatile.
2. Insoluble in water and soluble in organic solvents.
3. Most do not conduct electricity in any state.
This is because they do not have any free-moving ions or electrons to conduct electricity.
What is an ionic equation?
Most ionic compounds are soluble in water. They exist as ions in aqueous solution.
An ionic equation is a simplified chemical equation that shows the reactions involving ions in aqueous solution.
[Supplementary] Writing Ionic Equations
The reaction between aqueous silver nitrate and aqueous sodium chloride can be represented by the following equation:
silver nitrate + sodium chloride --> silver chloride + sodium nitrate
AgNO3(aq) + NaCl(aq) --> AgCl(s) + NaNO3(aq)
Silver nitrate, sodium chloride and sodium nitrate are soluble in water. They exist as ions in aqueous solution.
The chemical equation in terms of ions is:
Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) --> AgCl (s) + Na+(aq) + NO3-(aq)
The Na+ and NO3- ions are still ions at the end of the reaction as they have not taken part in the reaction. Such ions are called spectator ions.
Since only silver ions and chloride ions have reacted, the equation for the reaction can be simplified into the ionic equation below:
Ag+(aq) + Cl-(aq) --> AgCl (s)
An ionic equation shows the ions that take part in a reaction. It leaves out the spectator ions that do not react.