Electrolysis of Molten Sodium Chloride
Binary ionic compounds are made up of two types of elements only. In a molten binary ionic compound, there are only one type of positive ion and one type of negative ion.
During electrolysis using inert electrodes,
- Positive ions move to the cathode and gain electrons
- Negative ions move to the anode and lose electrons
A metal and a non-metal are formed as products.
Molten sodium chloride contains mobile sodium ions (Na+) and chloride ions (Cl-).
The figure below shows a setup of how electrolysis of molten sodium chloride is done. Click on the anode and cathode respectively to see the reactions and expected observations happening.
Reactivity Series and the Selective Discharge of ions
An aqueous solution of a compound is a mixture of the compound with water. Thus, it would contain:
1. The cation and anion from the dissolved substance
2. H+ and OH- from water
For example, an aqueous solution of NaCl contains sodium chloride and water. The ions present in the solution are ions from:
1. Sodium chloride, meaning Na+ and Cl-
2. The dissociation of water, meaning H+ and OH-
Thus, where there are two or more cations and anions in the solution, the ions are selectively discharged at both electrodes.
If inert electrodes are used during electrolysis, the ions discharged and hence products formed will depend on the following factors:
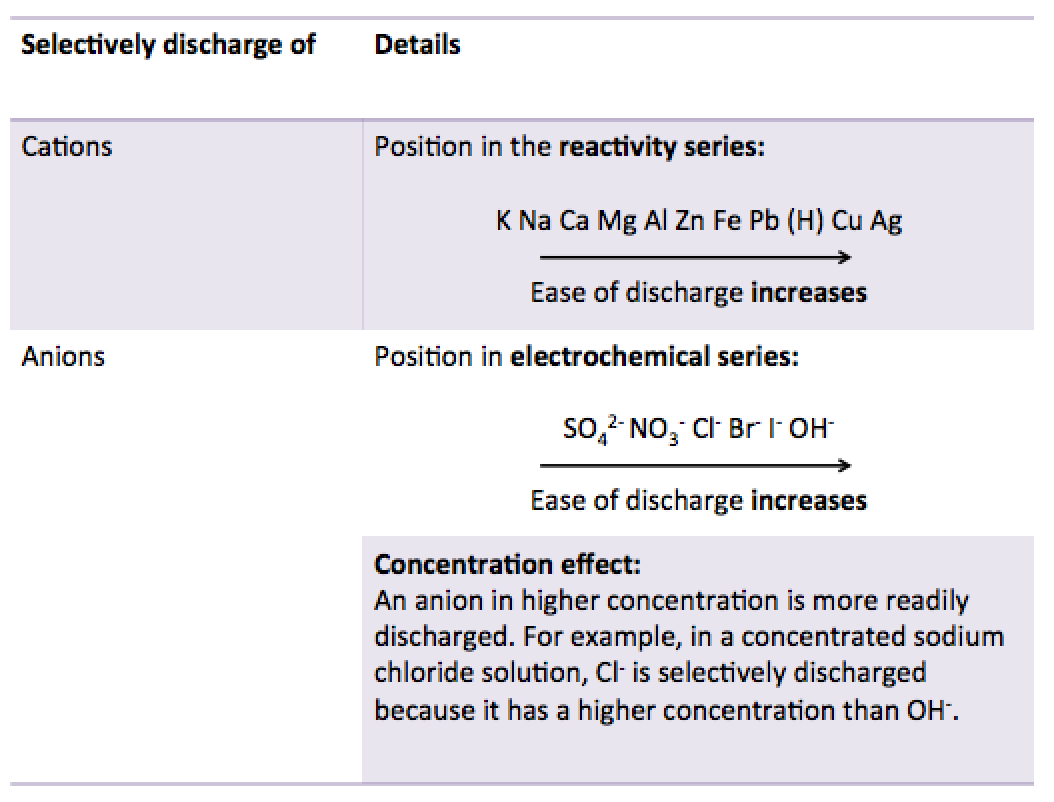
1. For cations, the position in the reactivity series.
2. For anions:
- The position in the electrochemical series (for dilute solutions)
- The concentration effect (for concentrated solutions)
General Rules
The following rules can be applied when predicting the products of electrolysis of any aqueous solution (using inert electrodes):
Electrolysis of Dil - Sodium Chloride Solution
Use
Electrolysis of Conc - Sodium Chloride Solution
Use
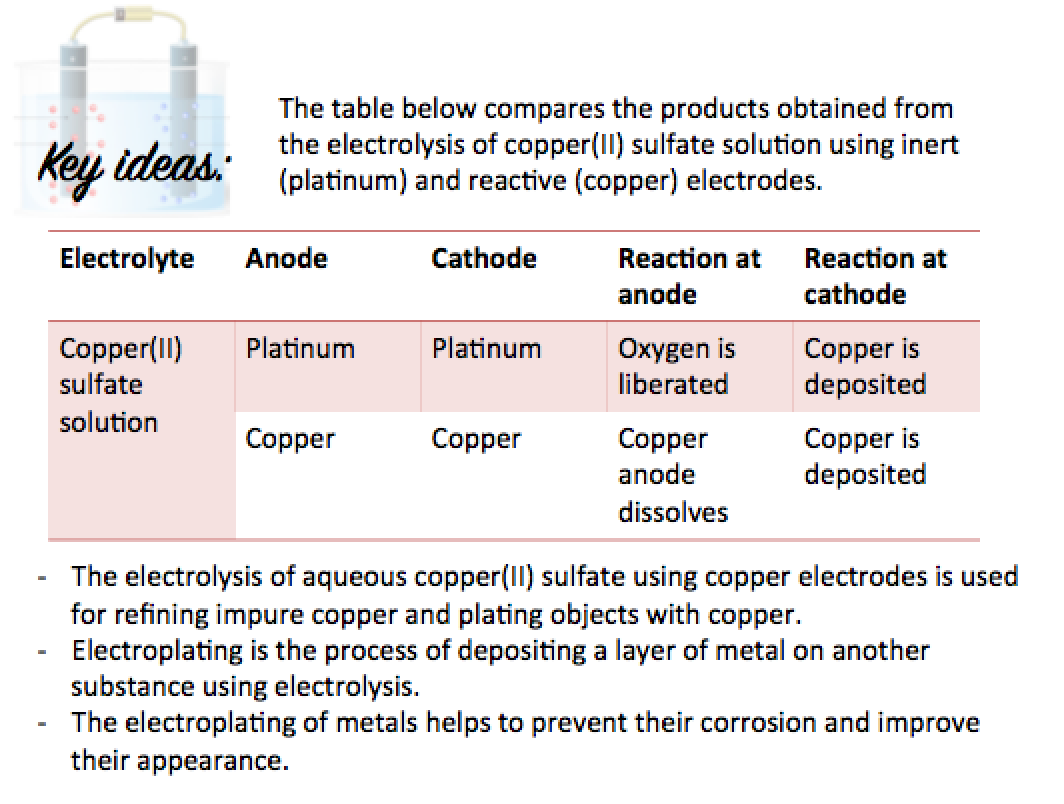
Summary
Here is a summary of the main points to note:
Test
Try out the following question involving a phosphoric acid fuel cell! Click on the Reveal Answer for each question after you have thought through it.