Energy Changes
In a chemical reaction, energy changes occur when energy is absorbed from the surroundings or released into the surroundings.
Endothermic: Energy is absorbed from the surroundings
Exothermic: Energy is released into the surroundings
A chemical reaction takes place when the starting materials, reactants, form new substances called the products. For reactants to form new substances (products), atoms in the reactants must absorb energy from its surroundings to break existing chemical bonds (an exothermic process), for the atoms to rearrange to form new bonds of the products.
Enthalpy Change
Since different chemical bonds have different bond energies, the amount of energy absorbed during bond-breaking and released during bond-formation will NOT be the same. Thus the energy content in the reactants and the products is different.
When a chemical reaction takes place, there is a net change of energy (enthalpy change) in the surroundings. It is measured in kilojoules (kJ) and represented by the symbol ?H.
The energy content of the reactants and products in a reaction can be represented by:
Hreactants --> energy content of the reactants
Hproducts --> energy content of the products
A positive sign (+) shows that energy has been absorbed from the surroundings.
A negative sign (-) shows that energy has been released into the surroundings.
The enthalpy change represents the difference in energy content of the reactants and products:
?H = total energy absorbed for bond breaking - total energy released for bond formation
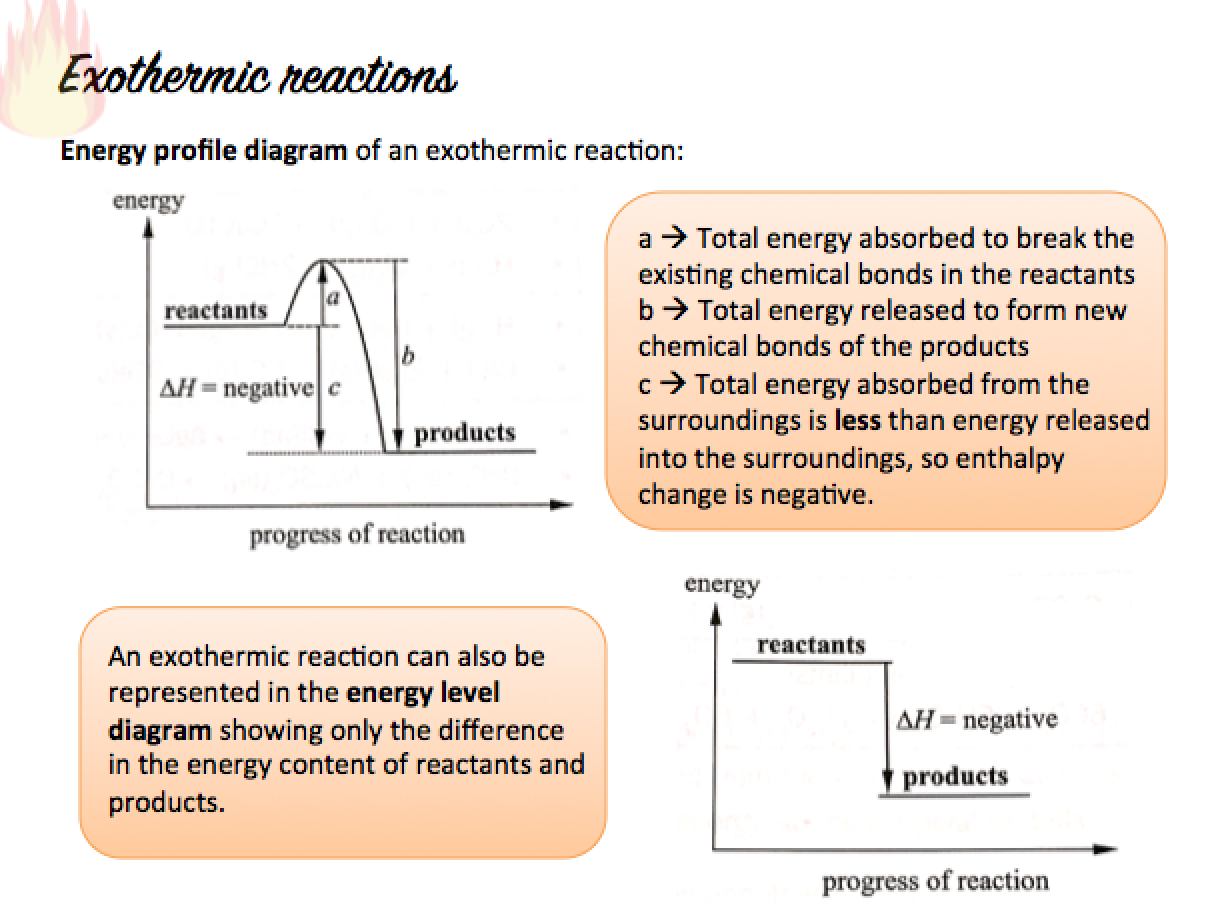
Exothermic Changes
An exothermic change is a change in which heat is given out to the surroundings.
When an exothermic change occurs,
- Heat is released from the reactants and transferred to the surroundings
- The temperature of the reaction mixture rises and the container feels warmer
In an exothermic reaction, the energy taken in during bond-breaking is less than the energy released during bond-formation. Hence, in an exothermic reaction:
- The energy content in the products is less that the energy content in the reactants.
- A net energy is released to the surroundings, so ?H is negative.
- The surroundings gain net energy, so temperature rises.
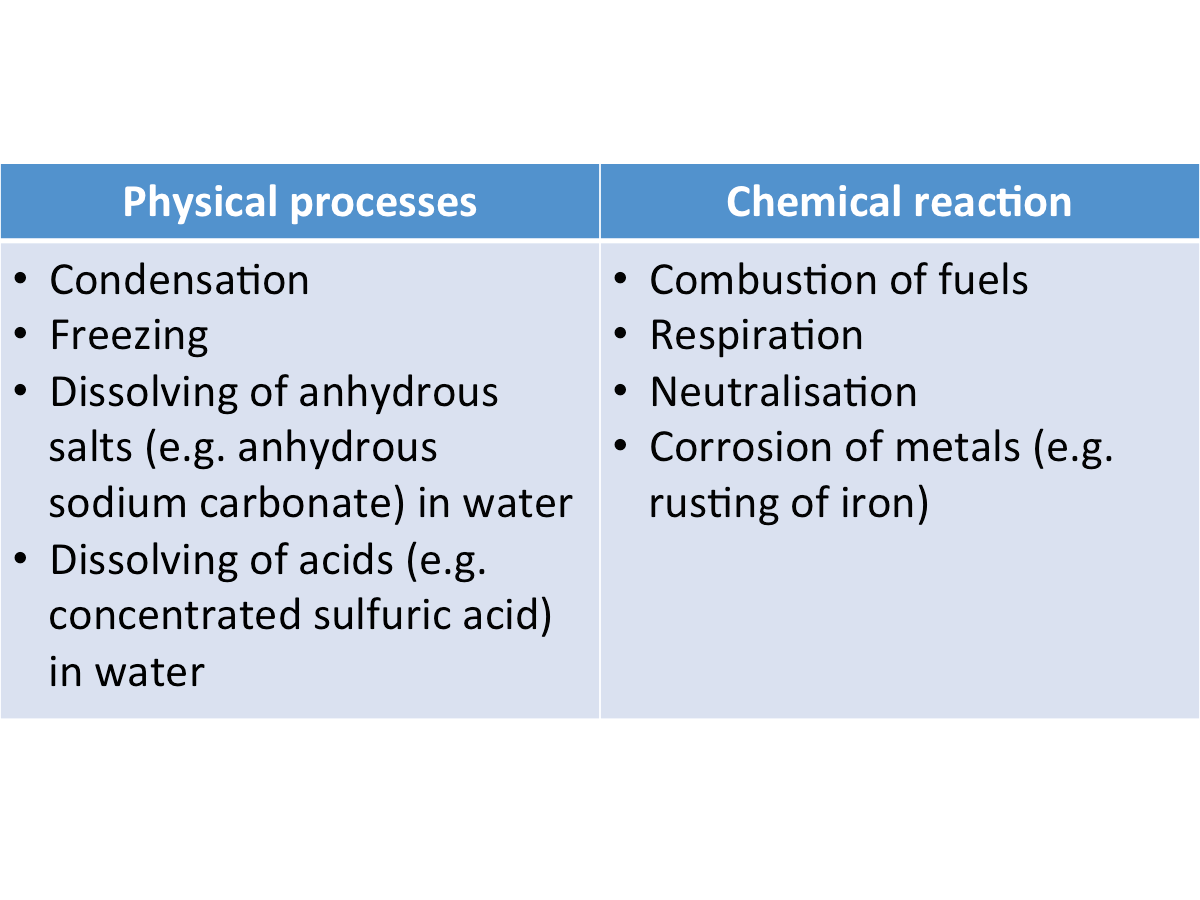
[Supplementary] Examples of Exothermic Changes
The table below shows some examples of exothermic changes.
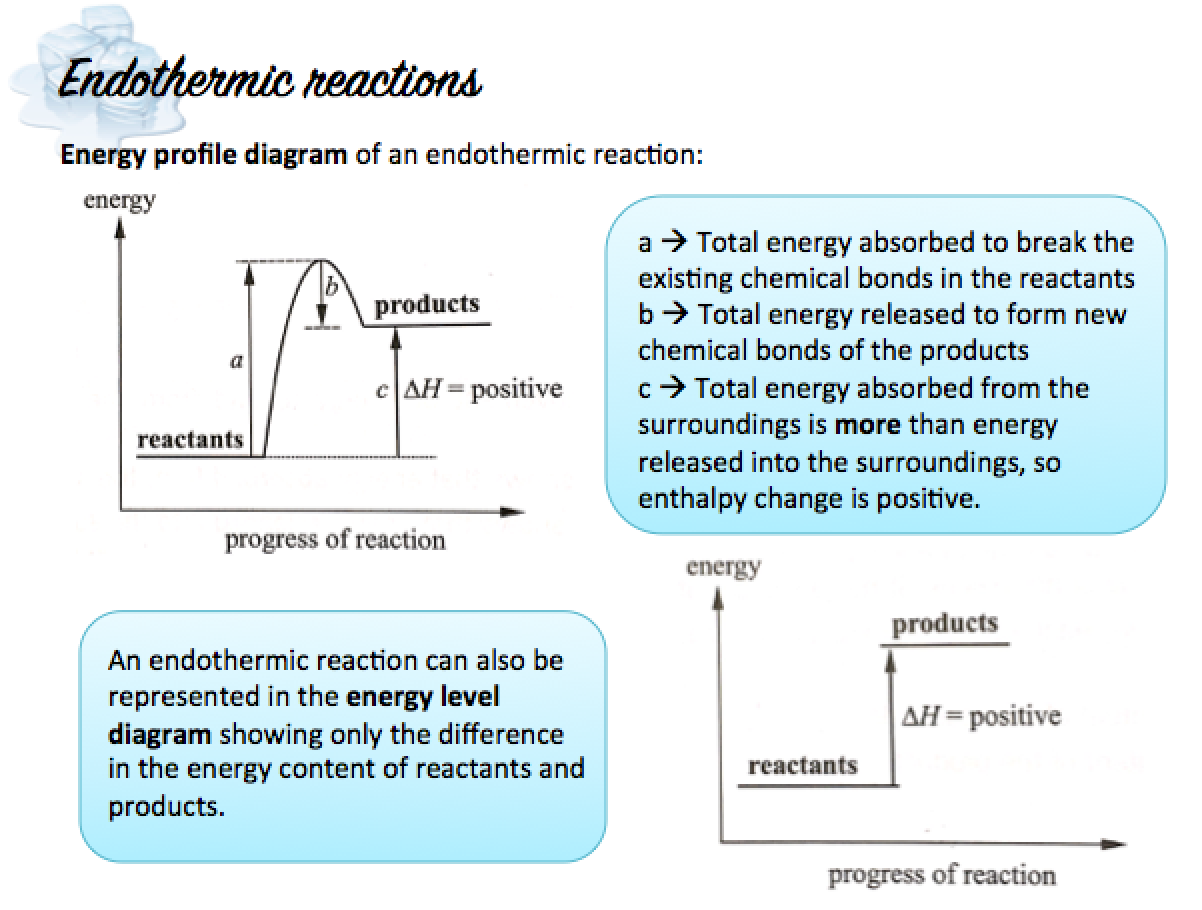
Endothermic Changes
An endothermic change is a change in which heat is absorbed from the surroundings.
When an endothermic change occurs,
- Heat energy is absorbed and transferred from the surroundings to the reactants
- The temperature of the reaction mixture falls and the container feels cooler
In an endothermic reaction, the energy taken in during bond-breaking is more than the energy released during bond-formation.
Hence, in an endothermic reaction:
- The energy content in the products is greater that the energy content in the reactants.
- A net energy is released to the surroundings, so ?H is positive.
- The surroundings gain net energy, so temperature falls.
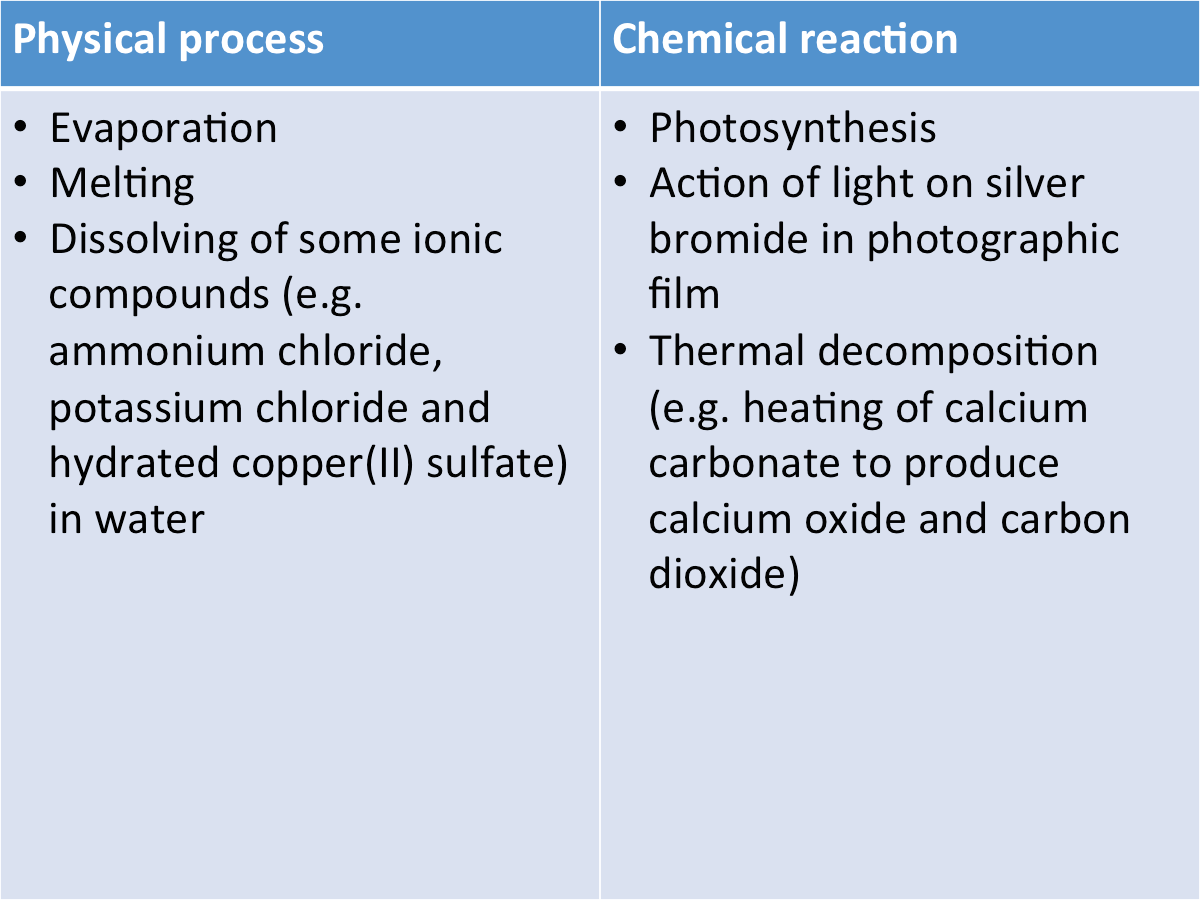
[Supplementary] Examples of Endothermic Changes
The table below shows some examples of endothermic changes.
[Supplementary] Calculation of Bond Forming and Bond Breaking
Bond energy is the energy required to break one mole of a specific covalent bond of a molecule. It is also the amount of energy released when one mole of a specific covalent bond is formed. It can be used to determine if the enthalpy change in a chemical reaction is exothermic or endothermic.
Below is an example of a question on enthalpy change:
[Supplementary] Activation Energy
Activation energy, Ea, is the minimum amount of energy that reactant particles must possess in order for a chemical reaction to occur.
- For a chemical reaction to take place, reactants must collide with each other. A reaction will only occur when the energy of the collision is greater than or equal to the required activation energy, where the covalent bonds will break. Otherwise, the molecules will just rebound.
- When a reaction has a low activation energy, the reaction can take place easily and spontaneously, as less energy is required to break the bonds. Similarly, when a reaction has a high activation energy, it does not take place easily as more energy is required to break the bonds.
In an energy profile diagram, the activation energy is the energy absorbed to break the chemical bonds. Watch the video below to learn more about activation energy.