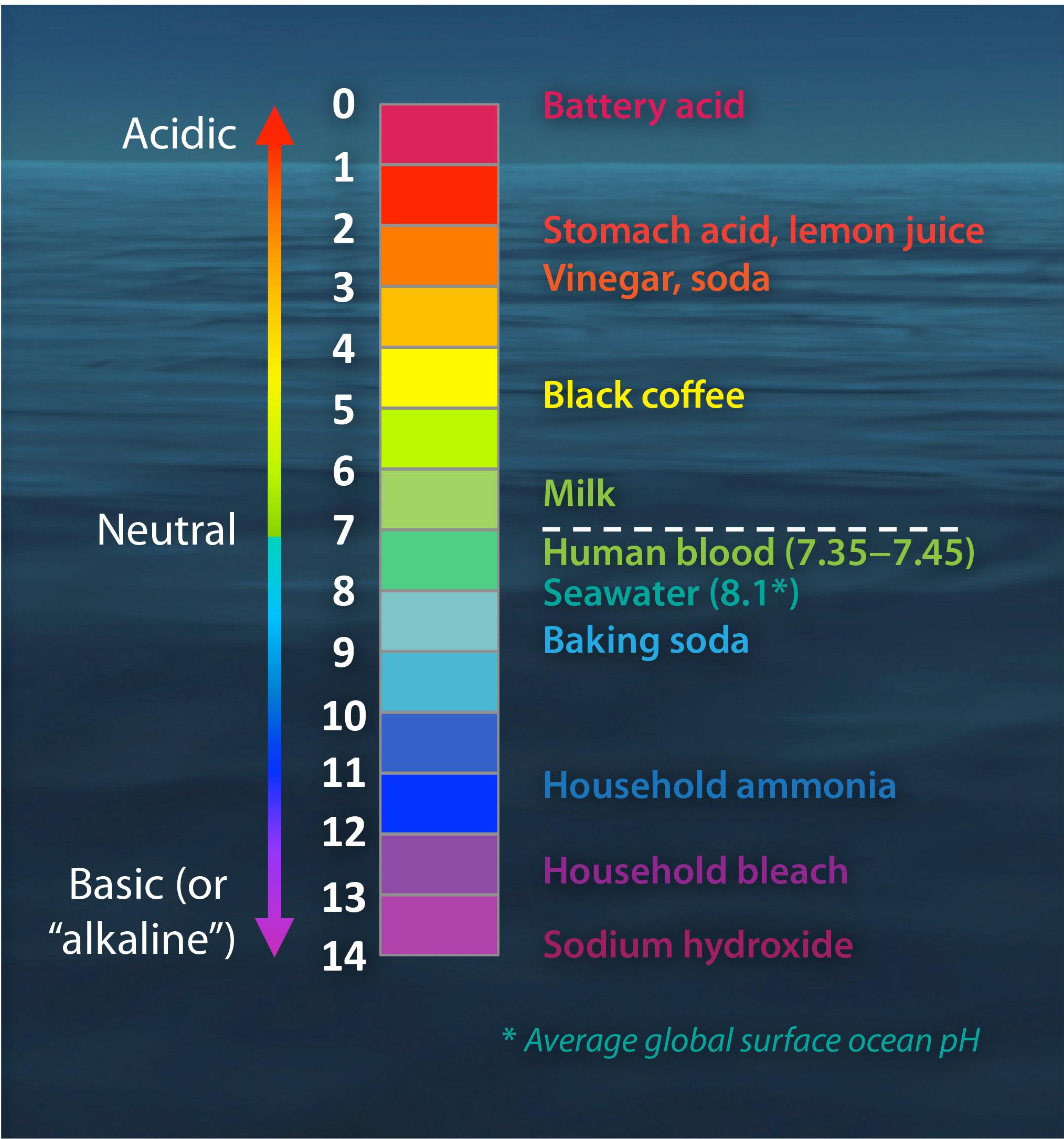
pH Scale
The pH scale is a set of numbers from 0 to 14 which is used to indicate whether a solution is acidic, neutral or alkaline.
- Acids have pH values less than 7.
- Alkalis have pH values greater than 7.
- A neutral solution has a pH value of exactly 7.
pH and Concentration of Hydrogen Ions
The pH of a solution is related to the concentration of hydrogen ions or hydroxide ions present in a solution.
The lower the pH value of a solution, the more acidic it is.
The higher the pH value of a solution, the more alkaline it is.
How can we measure the pH of a solution?
The pH of a given solution can be determined by using
- An indicator
- a pH sensor attached to a data logger
- pH meter
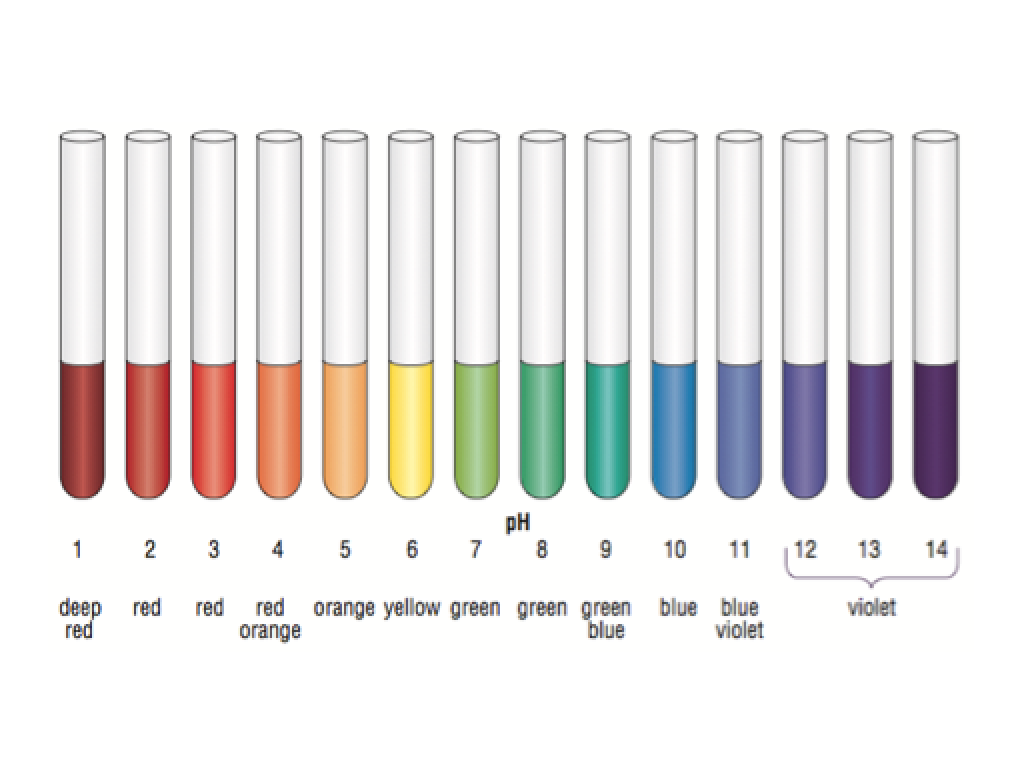
Universal Indicator
The pH value of a solution can be determined by using Universal Indicator. It comes in the form of a solution (or pH paper) and gives different colours in solutions of different pH.
Simply drop a few drops of universal indicator in a testube of pH to be determined and observe the results. The following image is a guide on what colour represents what pH:
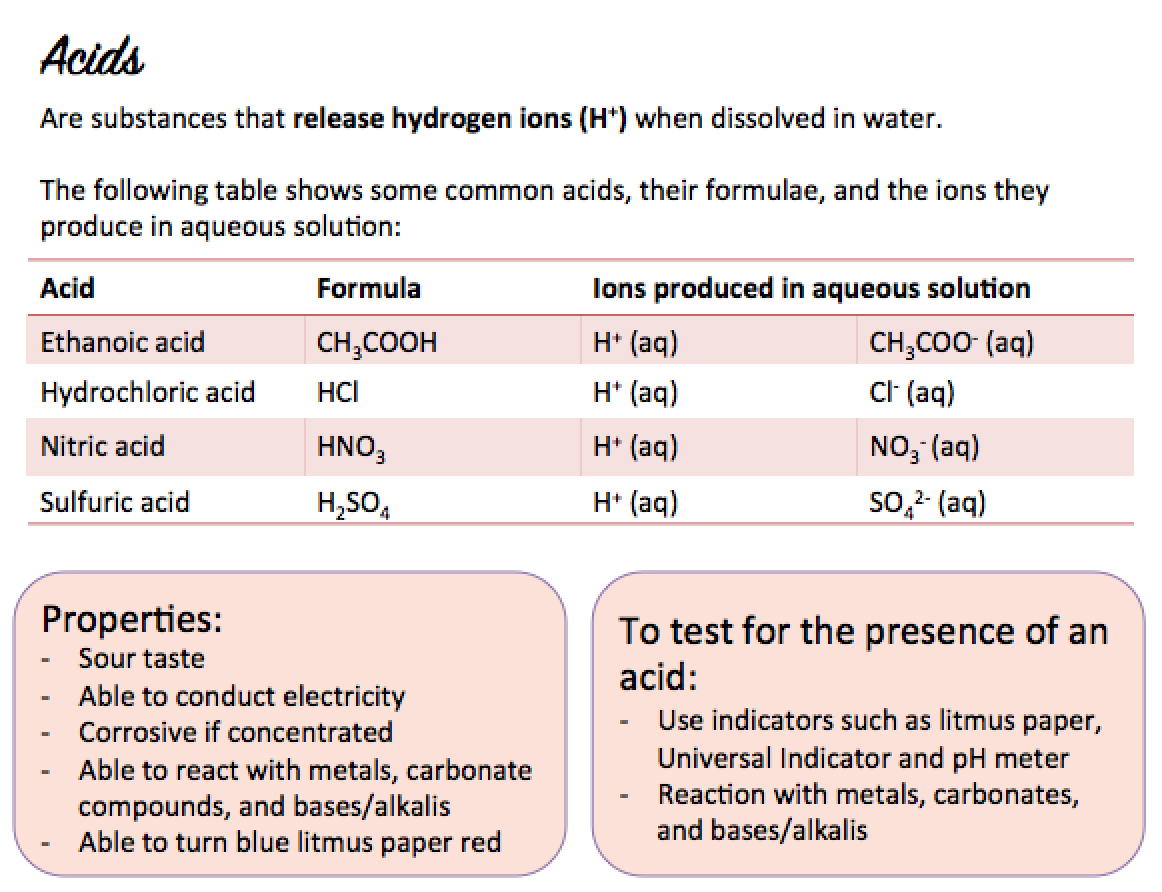
What is an acid?
An acid is a substance that produces hydrogen ions, H+, in aqueous solution.
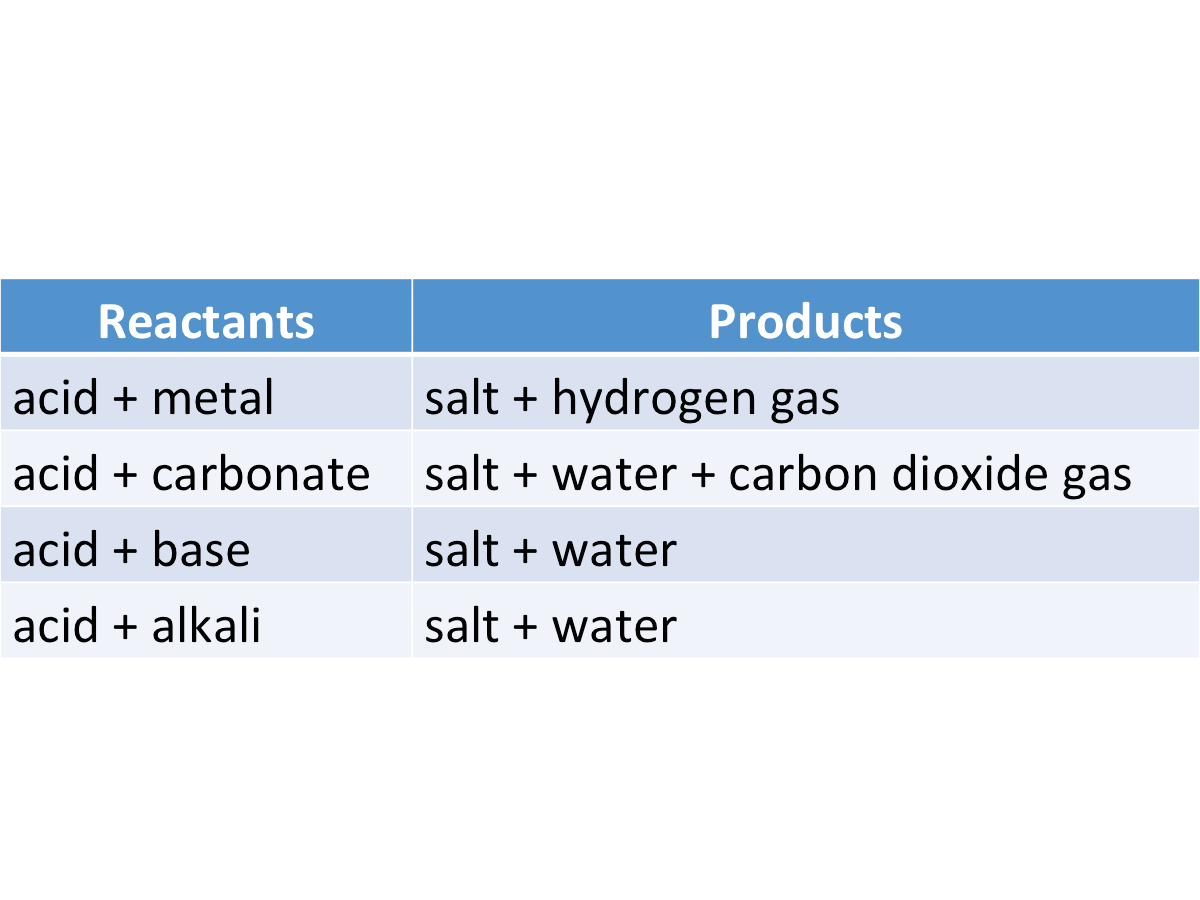
Reaction with Metals
Acids react with reactive metals to form hydrogen gas and a salt.
The general equation for the reaction is:
metal + acid --> salt + hydrogen
E.g. sodium + hydrochloric acid --> sodium chloride + hydrogen
2 Na (s) + 2 HCl (aq) --> 2 NaCl (aq) + H2 (g)
Reaction with Carbonates
Acids react with carbonates and hydrogen carbonates to form a salt, water and carbon dioxide.
General equation: carbonate + acid --> salt +water + carbon dioxide
The general equation for the reaction between acids and hydrogen carbonates is: hydrogen carbonate + acid ---> salt + water + carbon dioxide
We can test for carbon dioxide gas by bubbling the gas through limewater. Carbon dioxide forms a white precipitate with limewater. (Precipitate refers to insoluble solid particles produced in a liquid by chemical reactions.)
The video below demonstrates the reaction between an acid and a carbonate.
Reaction with Metal Oxides and Hydroxides
Acids react with metal oxides and hydroxides to form a salt and water only.
The general equation for the reaction of metal oxides with acid is:
metal oxide + acid --> salt + water
The general equation for the reaction of metal hydroxides with acid is:
metal hydroxide + acid --> salt + water
The video below shows how metal oxides react with acids to form a salt and water.
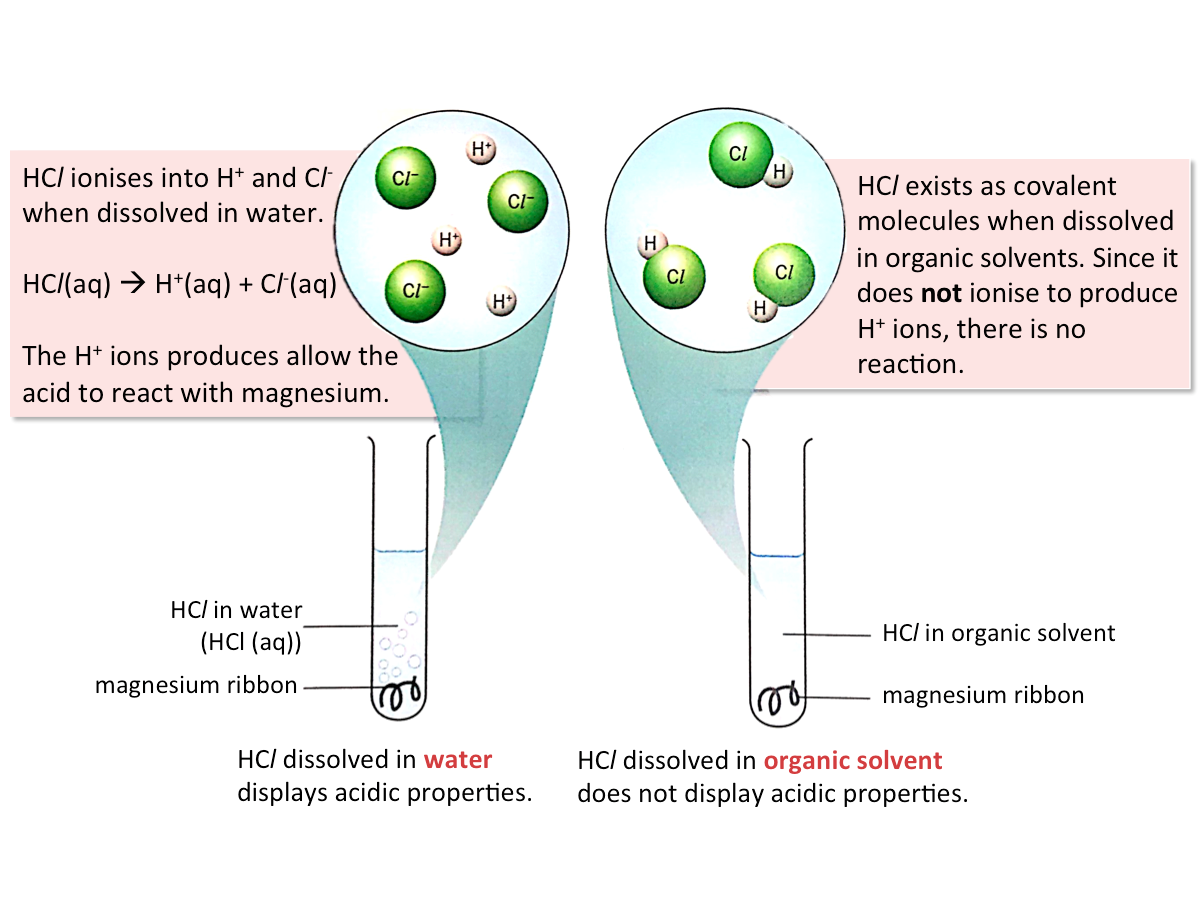
Role of Water in Acids
Acids only display their properties when dissolved in water, as they only produce hydrogen ions (H+) in water. The hydrogen ions give acids their acidic properties.
Uses of Acids
Sulfuric Acid
- Manufacture of fertilisers
- Manufacture of detergents
- Battery acid in cars
Hydrochloric Acid
- Cleaning impurities (e.g. rust from metals)
- Leather processing
Ethanoic Acid
- Vinegar (as food preservative and flavour enhancer)
- Manufacture of adhesives
Phosphoric Acid
- Flavouring in food and beverages
Test
Try out this true/false test on acids! Click on the red circle to view the answer for each question.
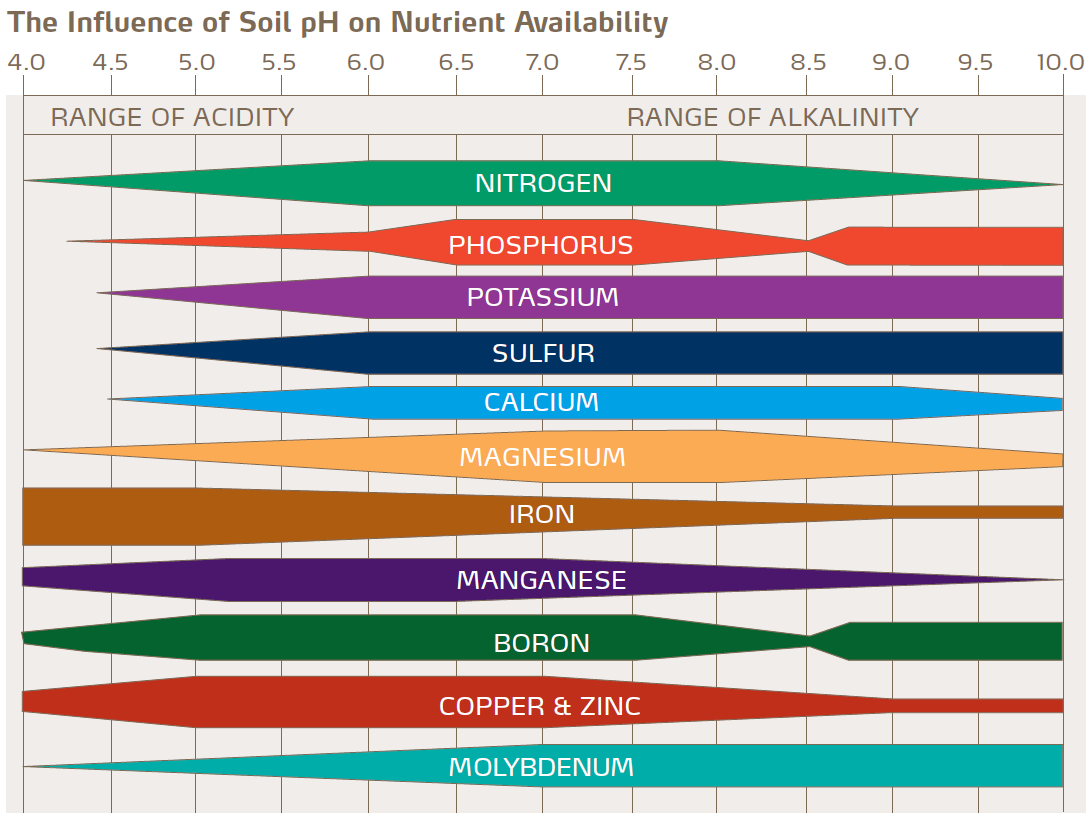
Why is soil pH important?
It is important to control the pH of soil as it affects the growth and development of plants. Most plants grow best when the pH is around 7.
How can we treat excess acidity?
Quicklime (calcium oxide) and slaked lime (calcium hydroxide) are often used to reduce the acidity of the soil. This is known as
What is a salt?
A salt is the ionic compound (meaning it contains a cation and an anion), formed when a metallic ion or an ammonium ion replaces one or more hydrogen ions of an acid. The table below shows reactions in which salts can be made.
Water of Crystallisation
Many salts combine with water molecules to form crystals. These water molecules are known as water of crystallisation, which can be removed by heating hydrated salts. Click on the blue boxes below to reveal explanations of what is happening at each point. The table below provides some examples of anhydrous and hydrated salts.
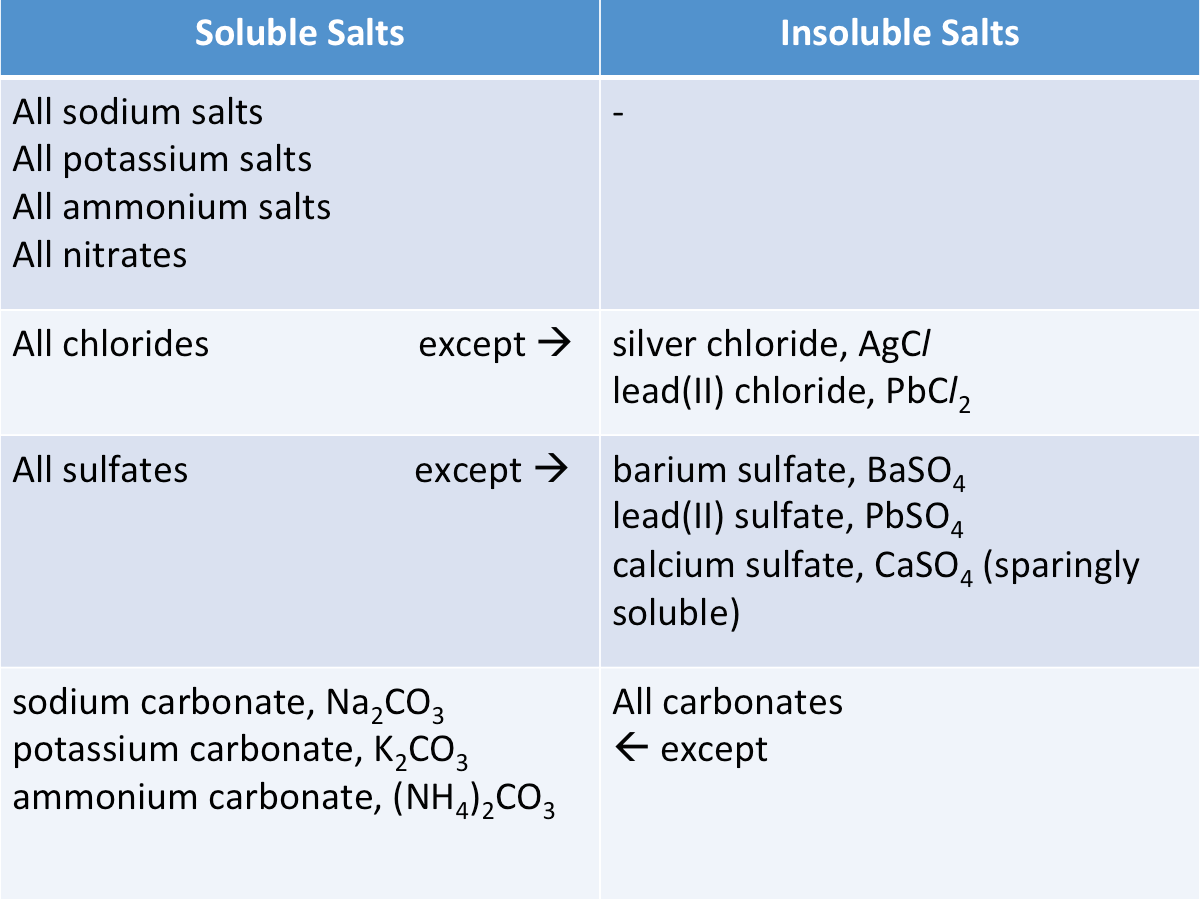
Soluble and Insoluble Salts
The table below shows the solubility of the common salts in water at room temperature.
Preparation of Salts
There are several ways to make salts. Two factors must be considered before deciding on how to prepare a salt:
1. Solubility of the salt
2. Solubility of the starting materialsIf the salt is soluble, it is usually prepared from the reaction with acids. If the salt is insoluble, it is usually prepared by the precipitation method.
The figure below gives an overview on the methods of preparing salts.
Method 1 (Soluble Salt): Reacting an Acid with Excess Metal, Insoluble Base or Insoluble Carbonate
The general equations for reacting an acid with a metal, insoluble base or insoluble carbonate are:
acid + metal --> salt + hydrogen gas
acid + insoluble base --> salt + water
acid + insoluble carbonate --> salt + water + carbon dioxide gas
Why must the metal, base or carbonate be in excess?
It is to ensure that all the acid is used up such that it will not contaminate the salt produced.
Why is it important that the metal, base or carbonate is insoluble in water?
This is such that the excess starting materials can be removed from the salt solution by filtration.
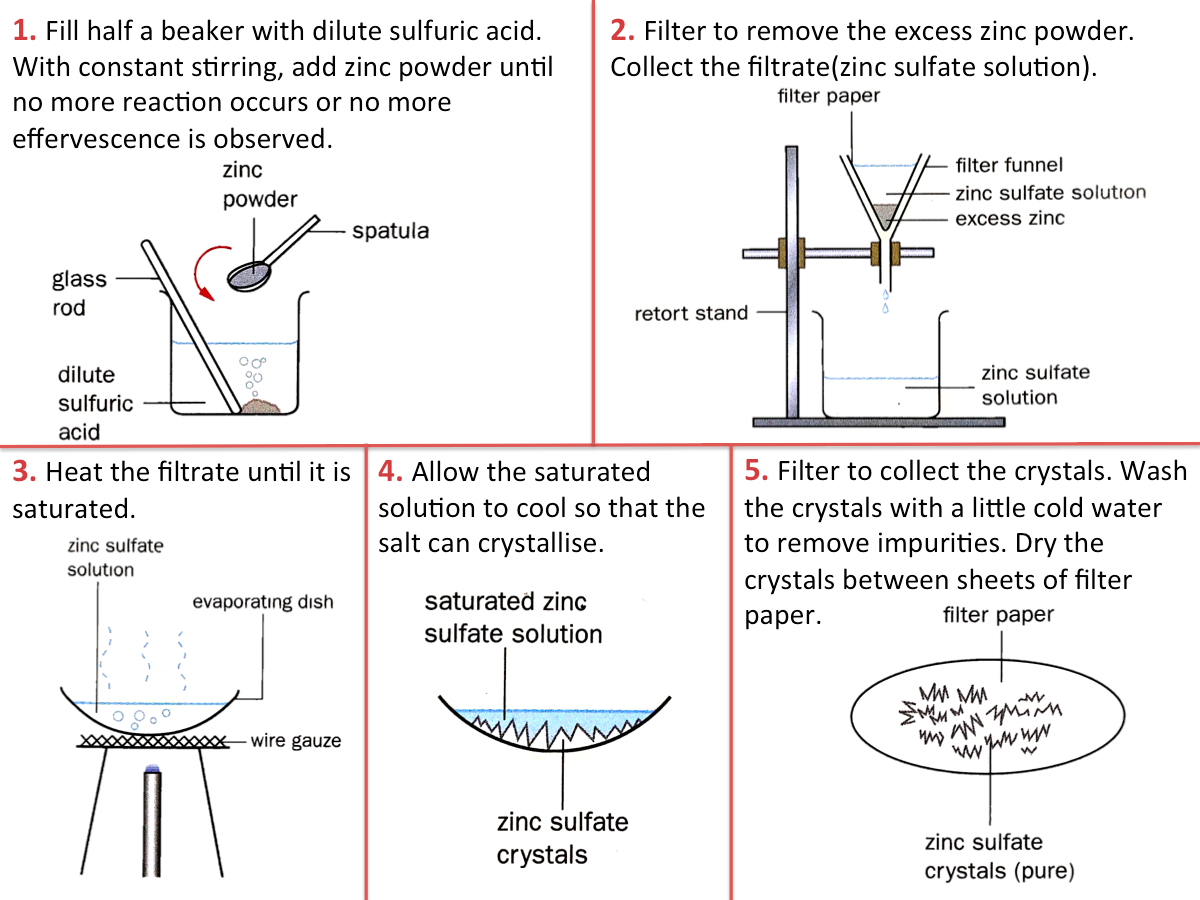
The figure below shows how zinc sulfate is prepared from the reaction of an acid with a metal.
Why is this method not suitable for some metals?
This method is suitable for moderately reactive metals such as zinc, magnesium and iron. However, it is not suitable for
- Very reactive metals (e.g. sodium, potassium, calcium), as they react violently with acids, making the reaction very dangerous.
- Unreactive metals (e.g. copper, silver)
For metals that are too reactive or unreactive, the salt can be prepared by reacting an acid with the insoluble base or carbonate instead. The apparatus and procedure used are similar to that shown in the figure above. The video below shows how copper(II) sulfate can be prepared by reacting an acid with an insoluble carbonate.
Reaction of Acid with Excess Insoluble Carbonate (Part 2)
The video below is a continuation of the above video.
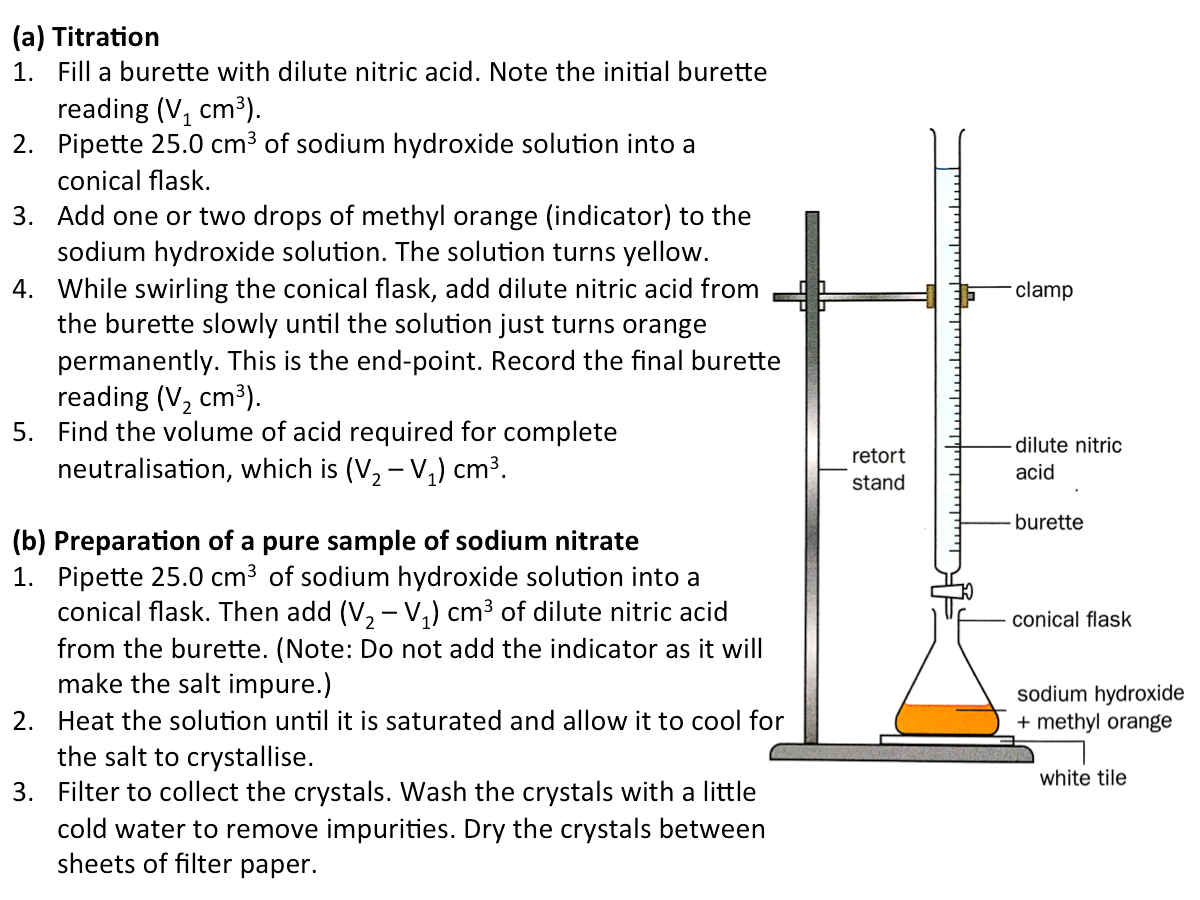
Method 2 (Soluble Salt): Titration
The general equations for the preparation of salts by titration is:
acid + alkali --> salt + water
acid + soluble carbonate --> salt + water + carbon dioxide
In this method, all the starting materials are soluble. They cannot be in excess as they cannot be removed from the salt solution by filtration and will cause the salt produced to be impure. Hence, the volume of each starting material used must be exact. Sodium, potassium and ammonium salts can be prepared using the titration method. The figure below shows how sodium nitrate can be prepared by titration.
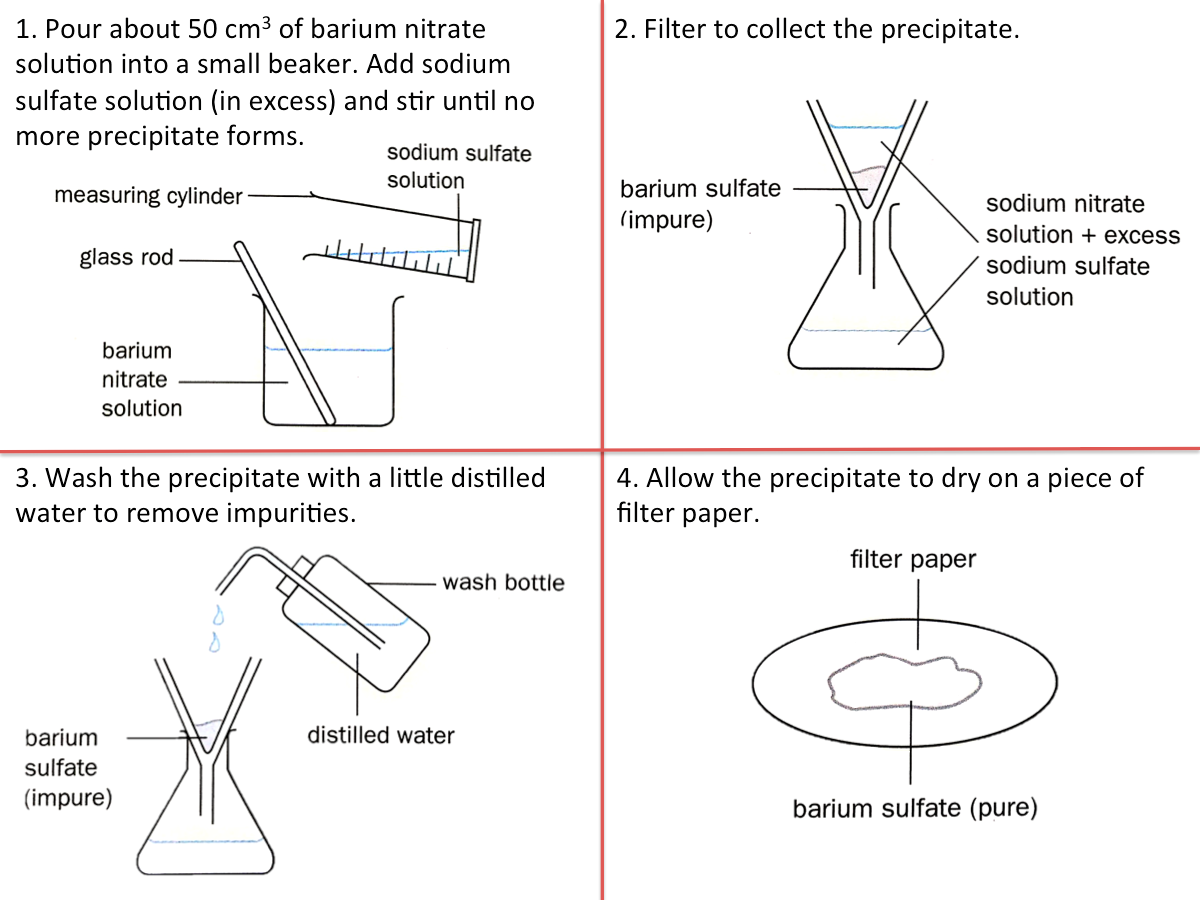
Method 3 (Insoluble Salt): Precipitation
Precipitation involves mixing two solutions to form an insoluble salt that separates out from the reaction mixture. The general equation for the preparation of salts by precipitation is:
aqueous solution AB + aqueous solution XY --> insoluble salt AY + aqueous solution XB
This method is used for preparing insoluble salts since the salts can be separated from the starting materials by filtration. The figure below shows how barium sulfate can be prepared by precipitation.
What is qualitative analysis?
Qualitative analysis is the process of identifying dissolved cations and anions in a solution by adding certain reagents to the solution and observing the changes that occur.
The video below gives an overview of how certain cations are identified using qualitative analysis. Take note of the colours of the solutions and precipitates formed in the tests.
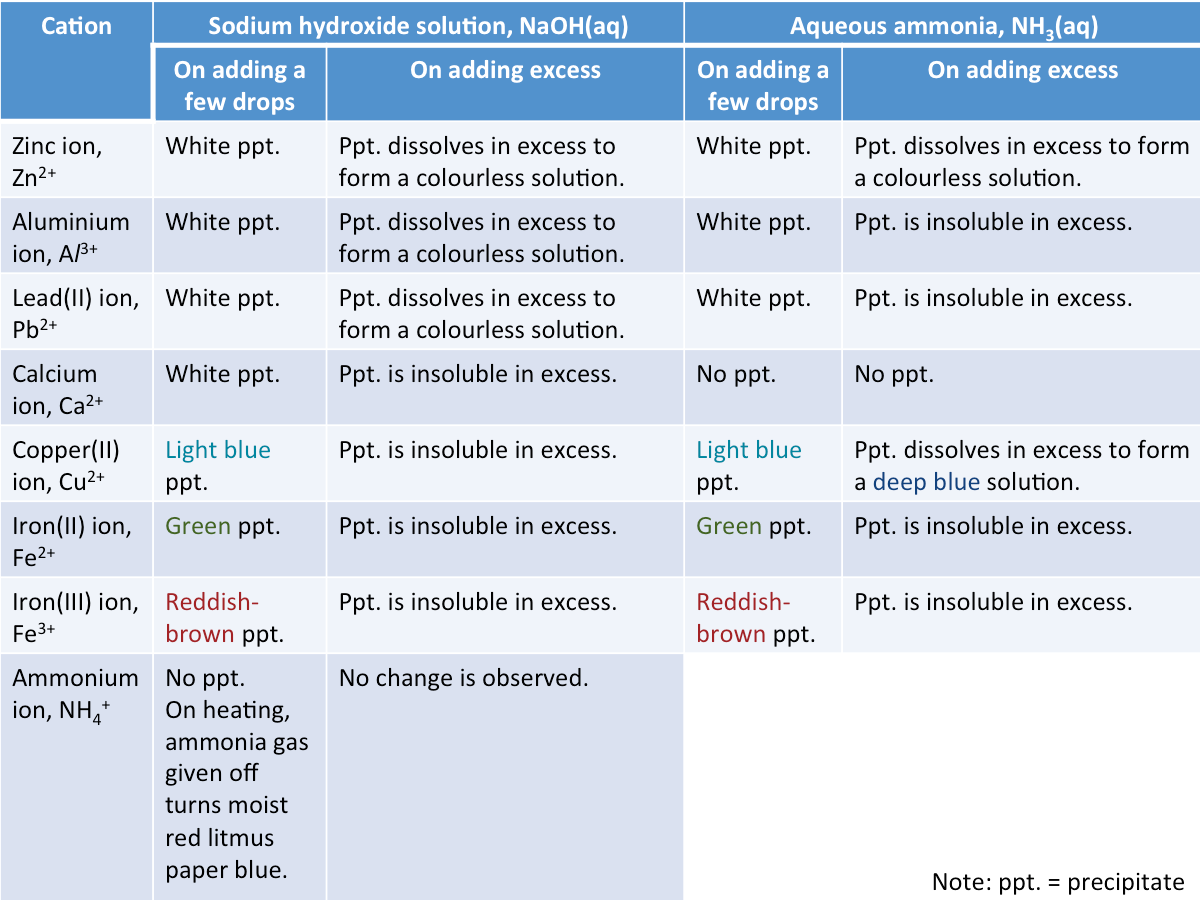
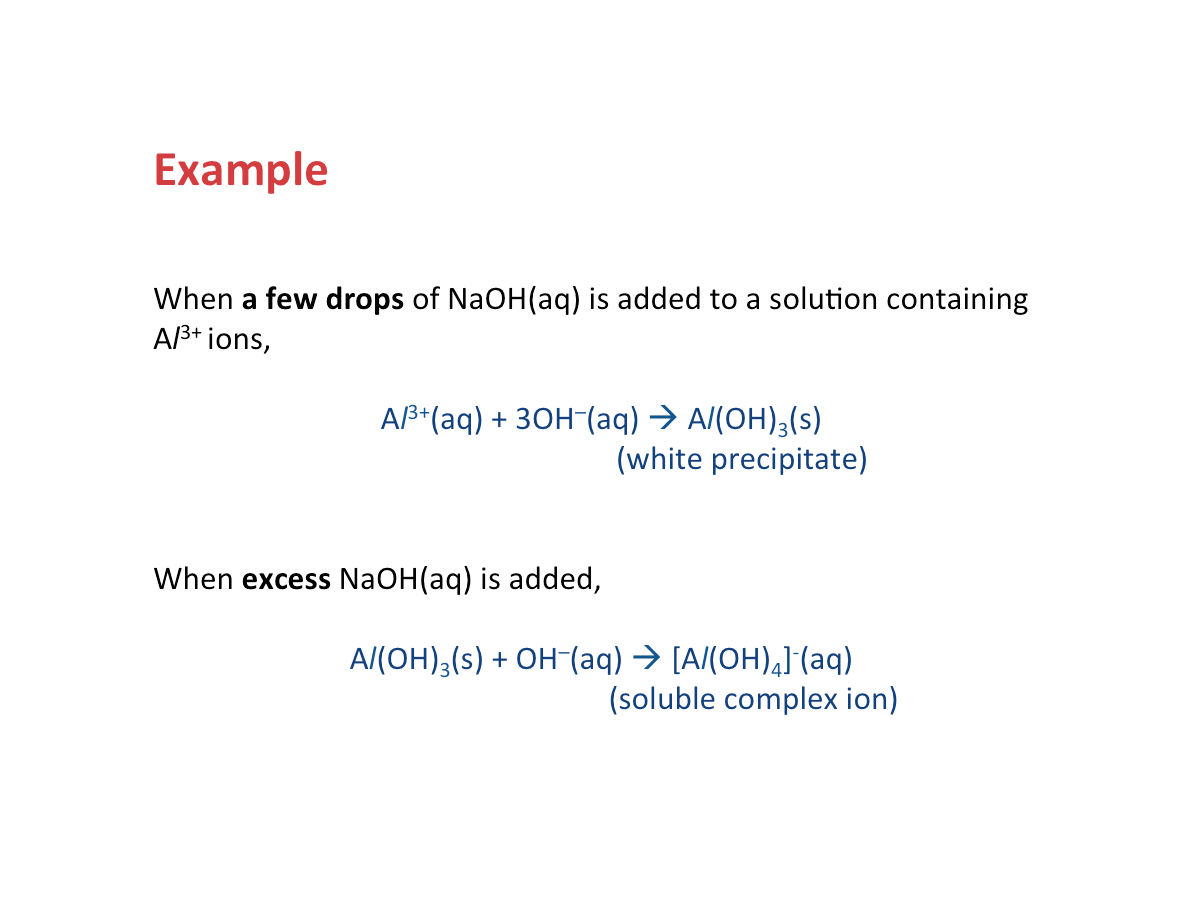
How are cations identified?
The reagents used to identify cations are sodium hydroxide solution, NaOH(aq), and aqueous ammonia, NH3(aq).
There are two stages for adding NaOH(aq) or NH3(aq) to the solution containing cations:
Step 1: Add a few drops
Step 2: Add excess
At each step, look out for:
1. Colour of the precipitate
2. Whether or not the precipitate dissolves in excess NaOH(aq) or NH3(aq)
The table below summarises what would be observed when sodium hydroxide solution and aqueous ammonia are added to solutions containing different cations.
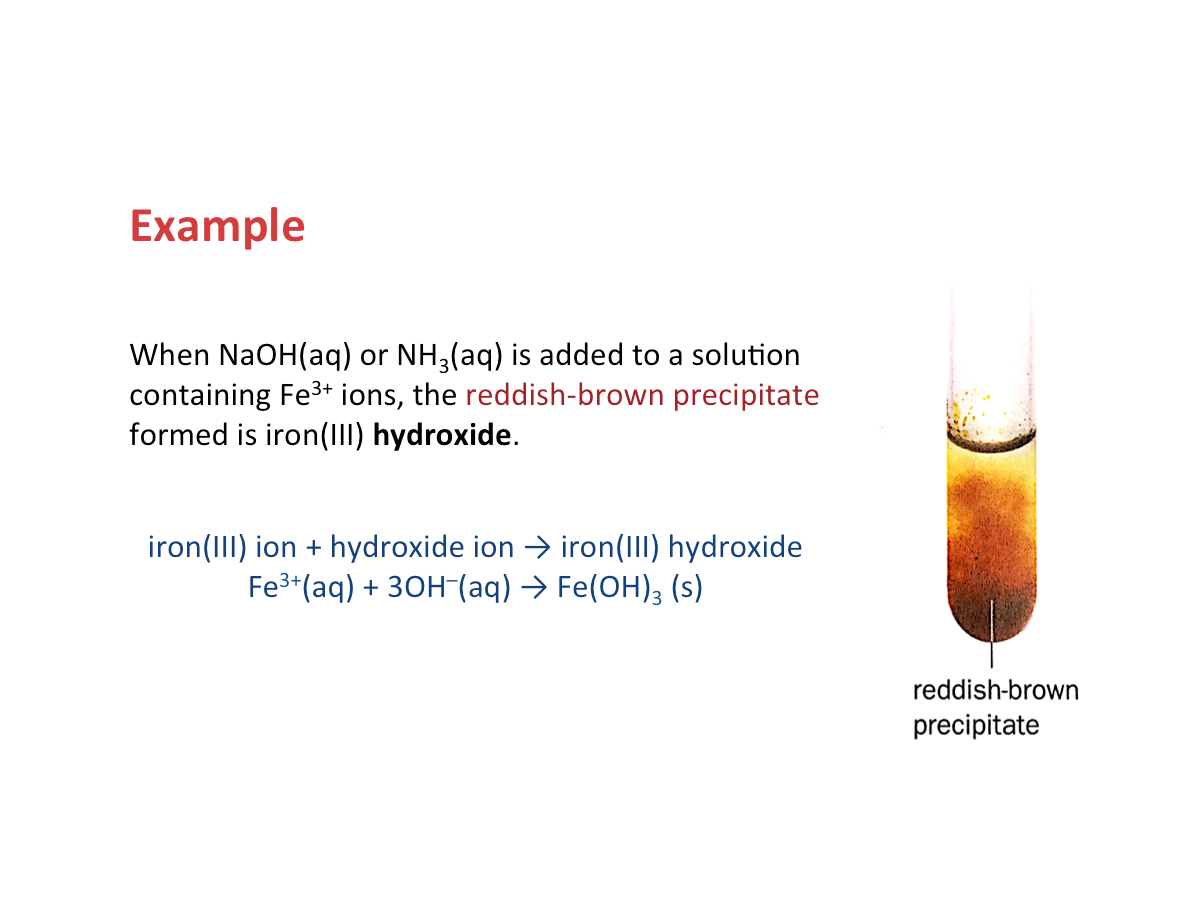
Why do we get precipitates upon adding the reagents?
The precipitate formed in each reaction is the hydroxide of the metal ion.
Why do some precipitates dissolve in excess of the reagent?
This is due to the formation of compounds that are soluble in water.
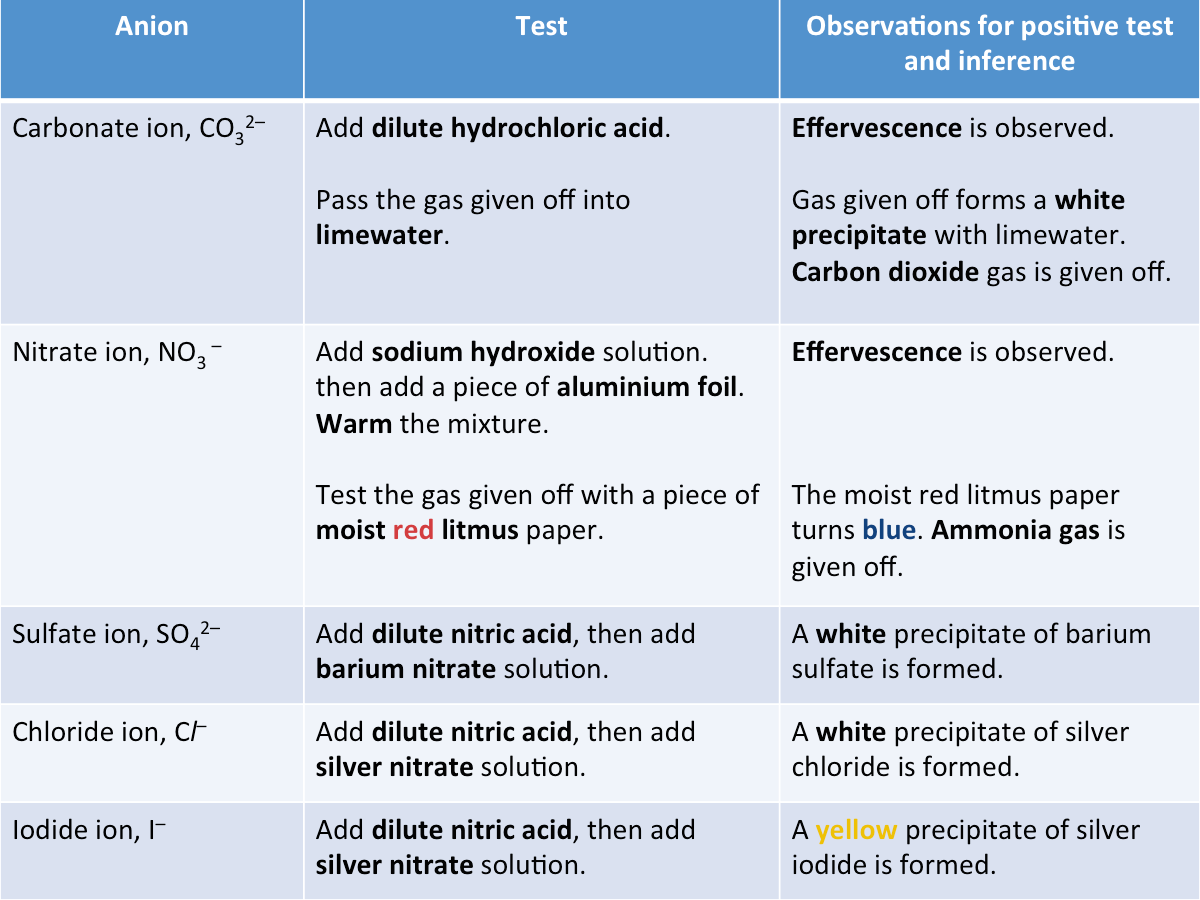
How are anions identified?
The tests shown in the table below can be used to identify anions.
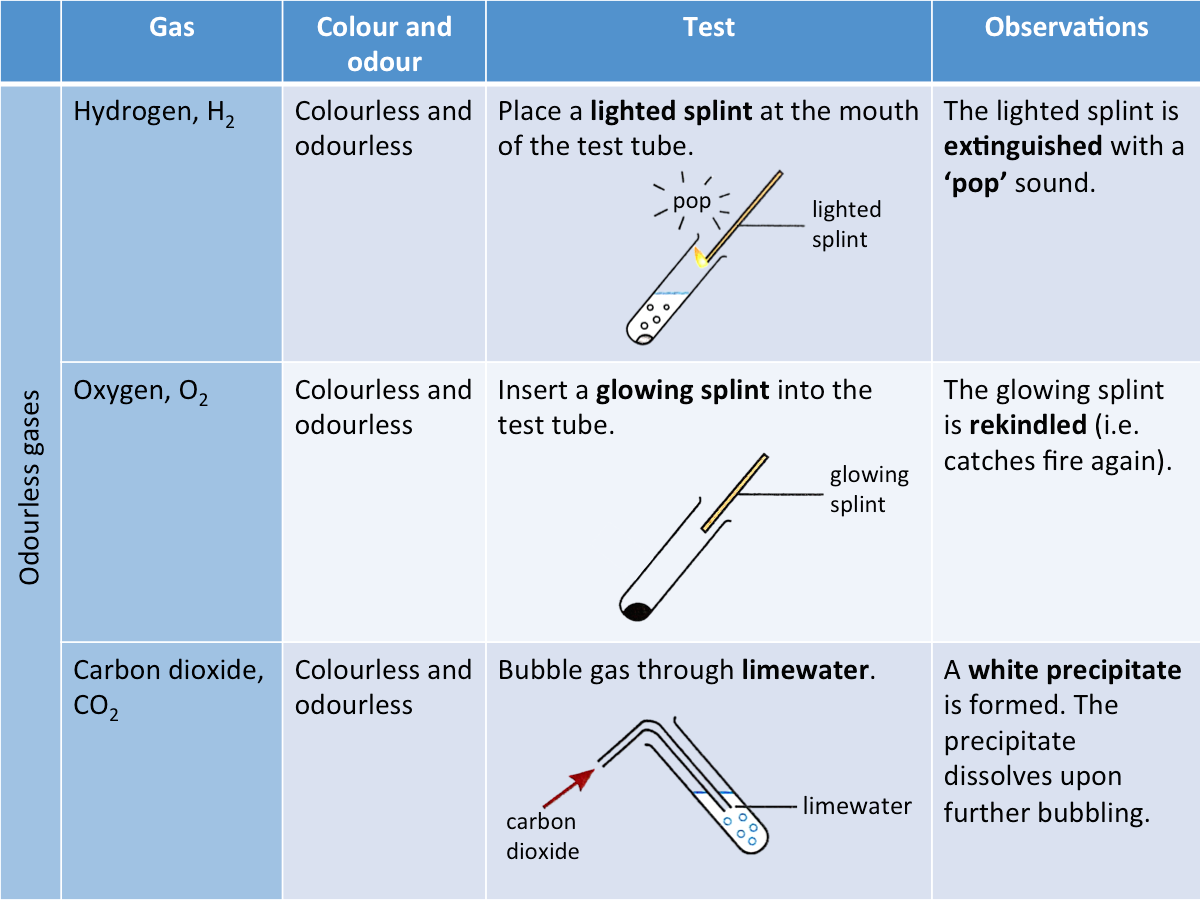
How are odourless gases identified?
A gas is often liberated when an unknown salt is being tested. The table below shows the tests used to identify some common odourless gases.
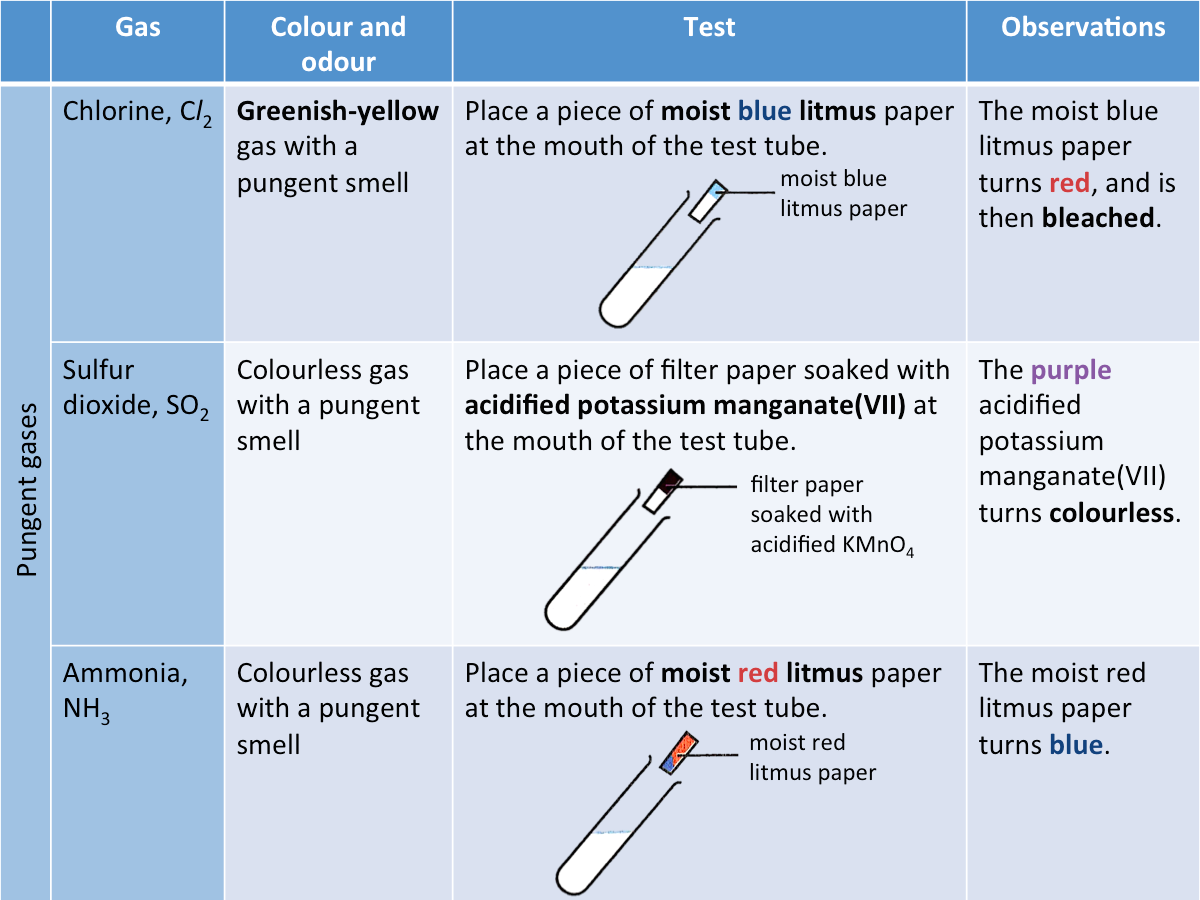
How are pungent gases identified?
The table below shows the tests used to identify some common pungent gases.