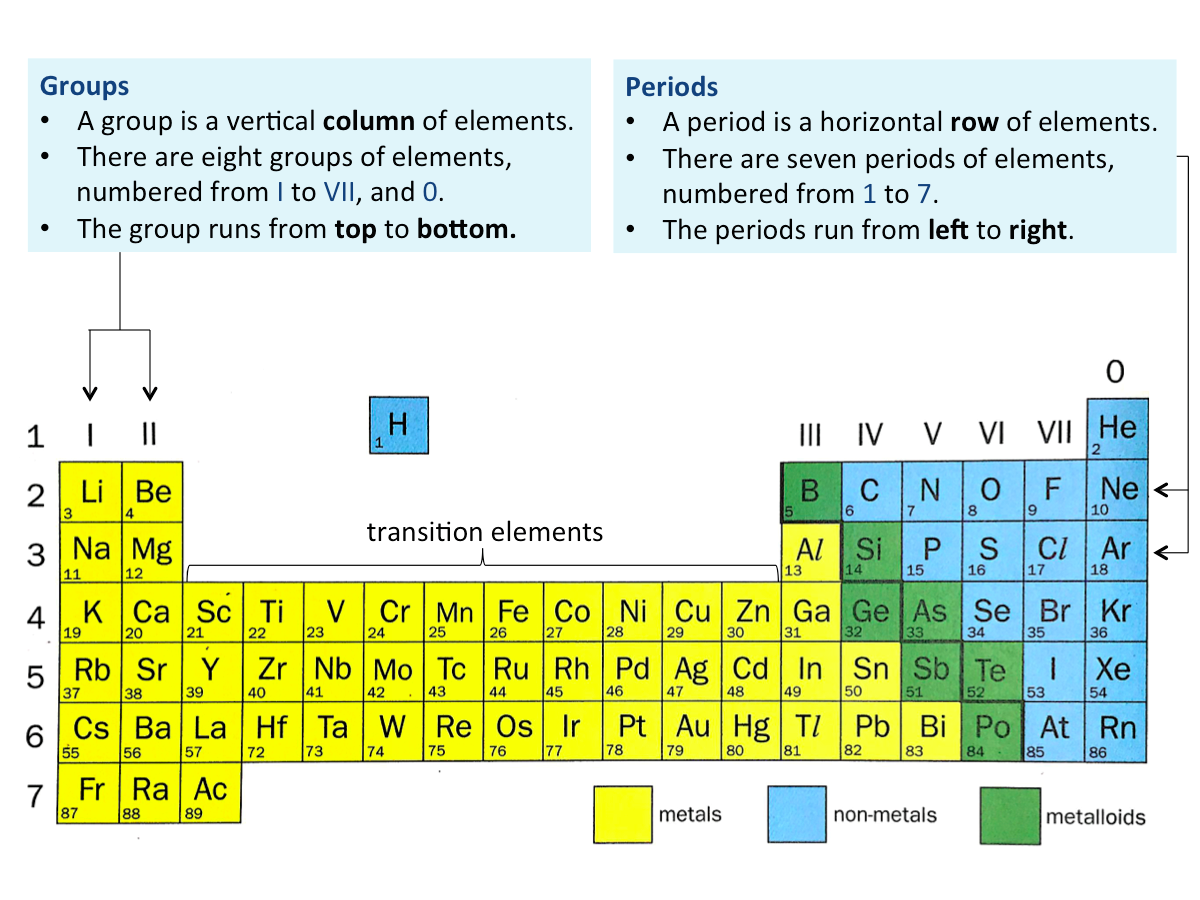
What are the features of the periodic table?
The periodic table is a list of elements arranged in order of increasing proton (atomic) numbers.
The Periodic Table divides the elements into periods and groups.
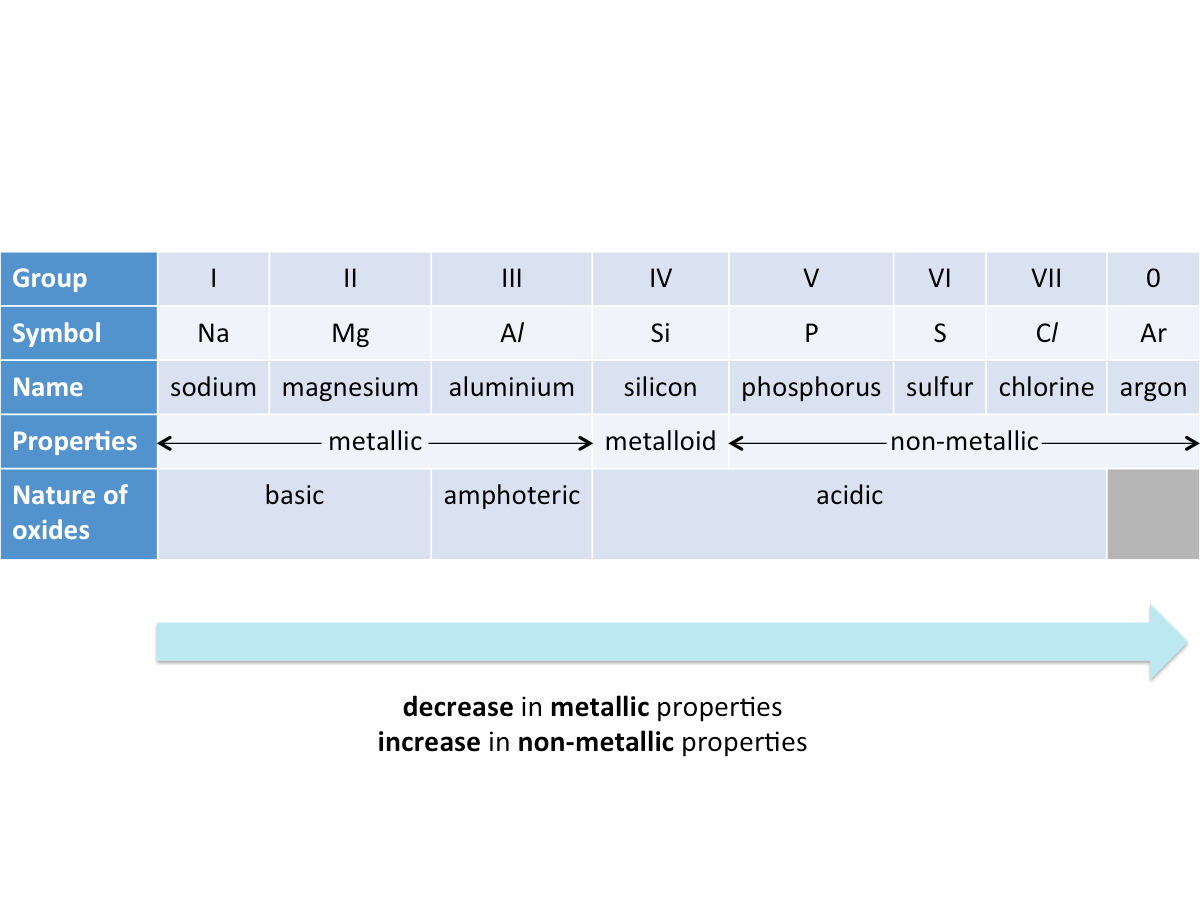
How do metallic properties of elements change across a period?
From left to right across the period, there is a decrease in metallic properties and an increase in non-metallic properties.
How do the metallic properties of elements change down a group?
Going down a group,
- Size of atom increases
- Valance electrons are further away from the attractive force of the nucleus
Hence an element further down a group will lose its valence electron more easily.
Going down a group, there is an increase in metallic properties and a decrease in non-metallic properties.
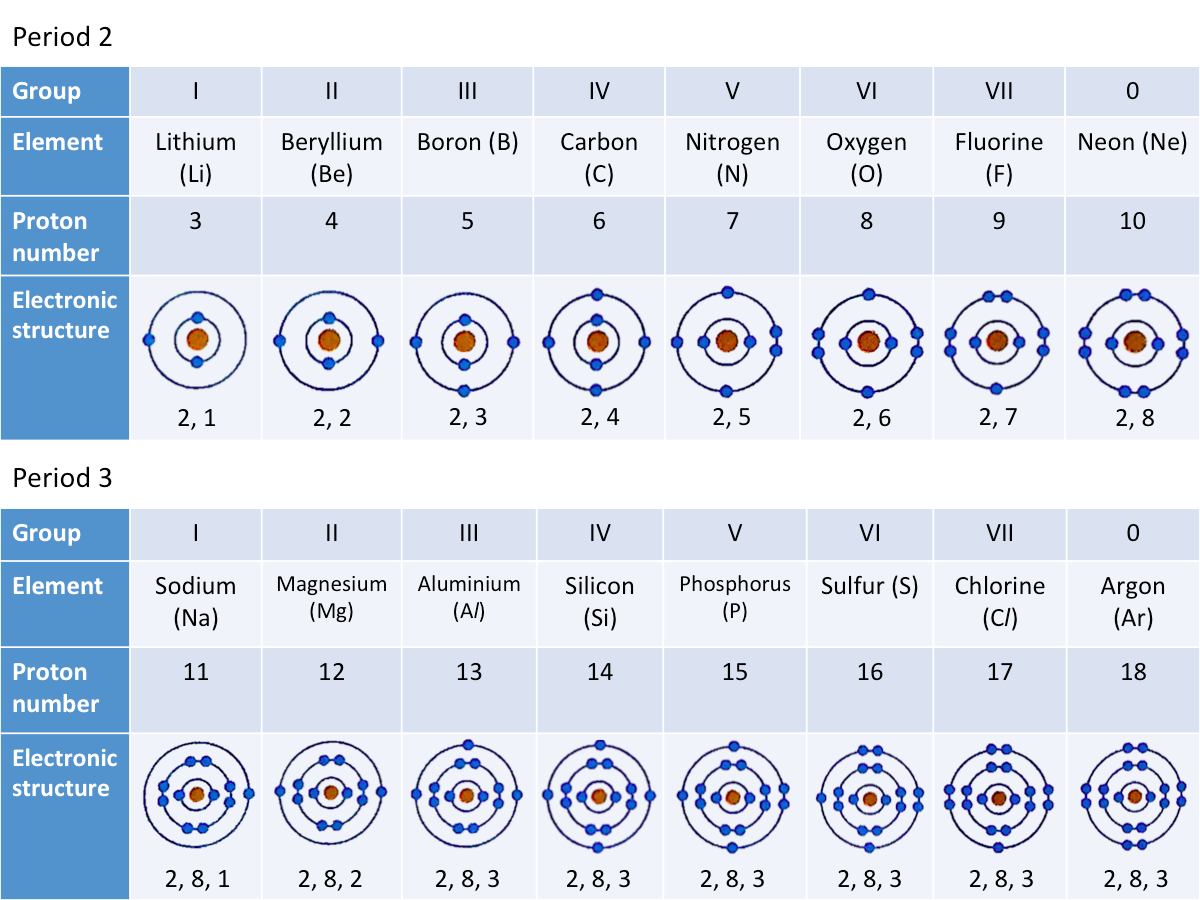
Electronic Structure
The electronic structure of an element can be obtained from its proton number. From this, the period number and group number of the element can be deduced.
The electronic structures of the elements of Periods 2 and 3 are shown in the table below.
How are elements in the same period similar in terms of their electronic structures?
If you look at the table above, you will notice that
- Elements of Period 2 have two electron shells
- Elements of Period 3 have three electron shells
The number of electron shells is the same as the period number of the element.
How are elements in the same group similar in terms of their electronic structures?
Looking at the table again, you will notice that
- Group I elements have one valence electron
- Group V elements have five valence electrons
The number of valence electrons is the same as the group number of the element.
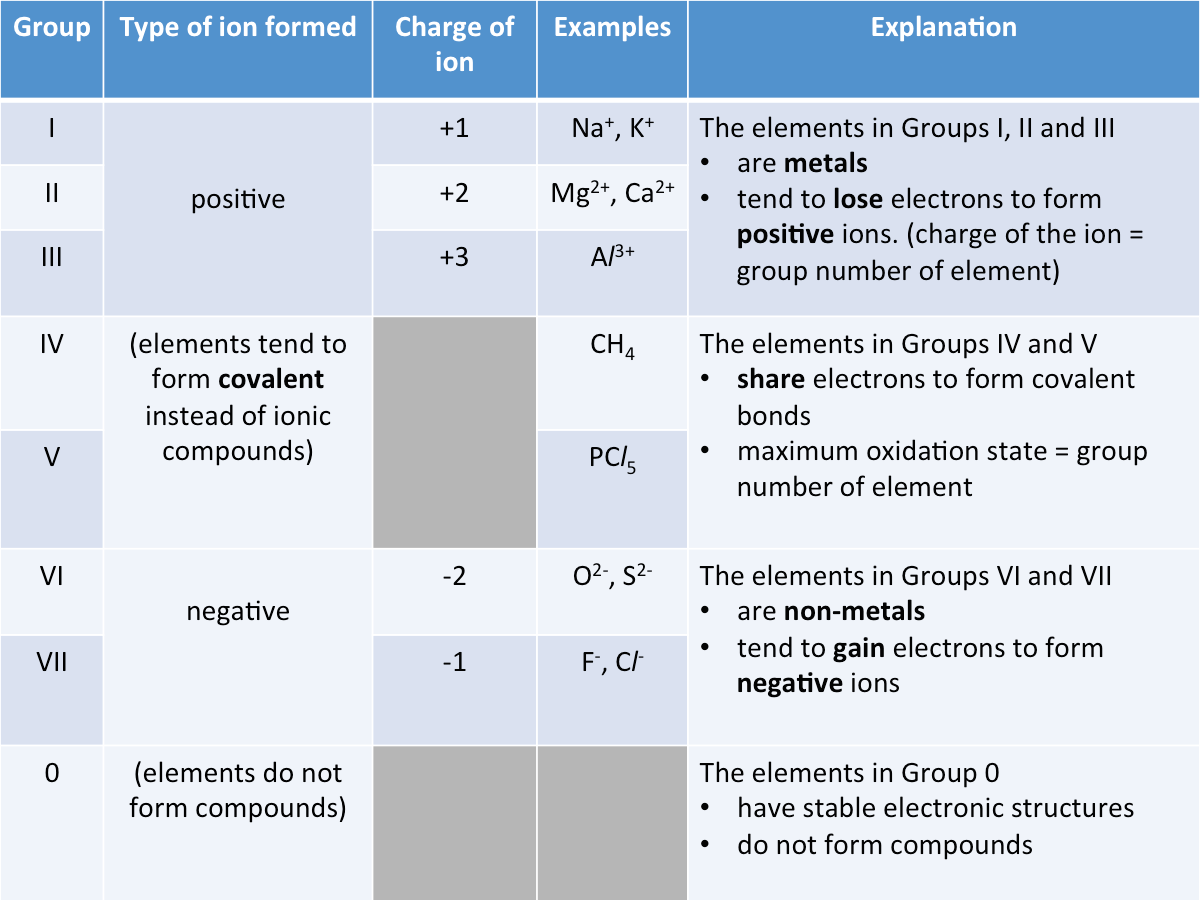
Charge of Ion
The table below shows the relationship between the group number and the charge of the ion formed by each element.
What are alkali metals?
The elements in Group I of the Periodic Table are alkali metals. They have similar properties since they belong to the same group.
Watch the video below to learn about the properties of alkali metals.
Physical Properties of Alkali Metals
Alkali metals
- Are soft and can be cut easily
- Have low melting and boiling points
- Have low densities (lithium, sodium and potassium float on water)
How do the physical properties change going down the group?
Down the group,
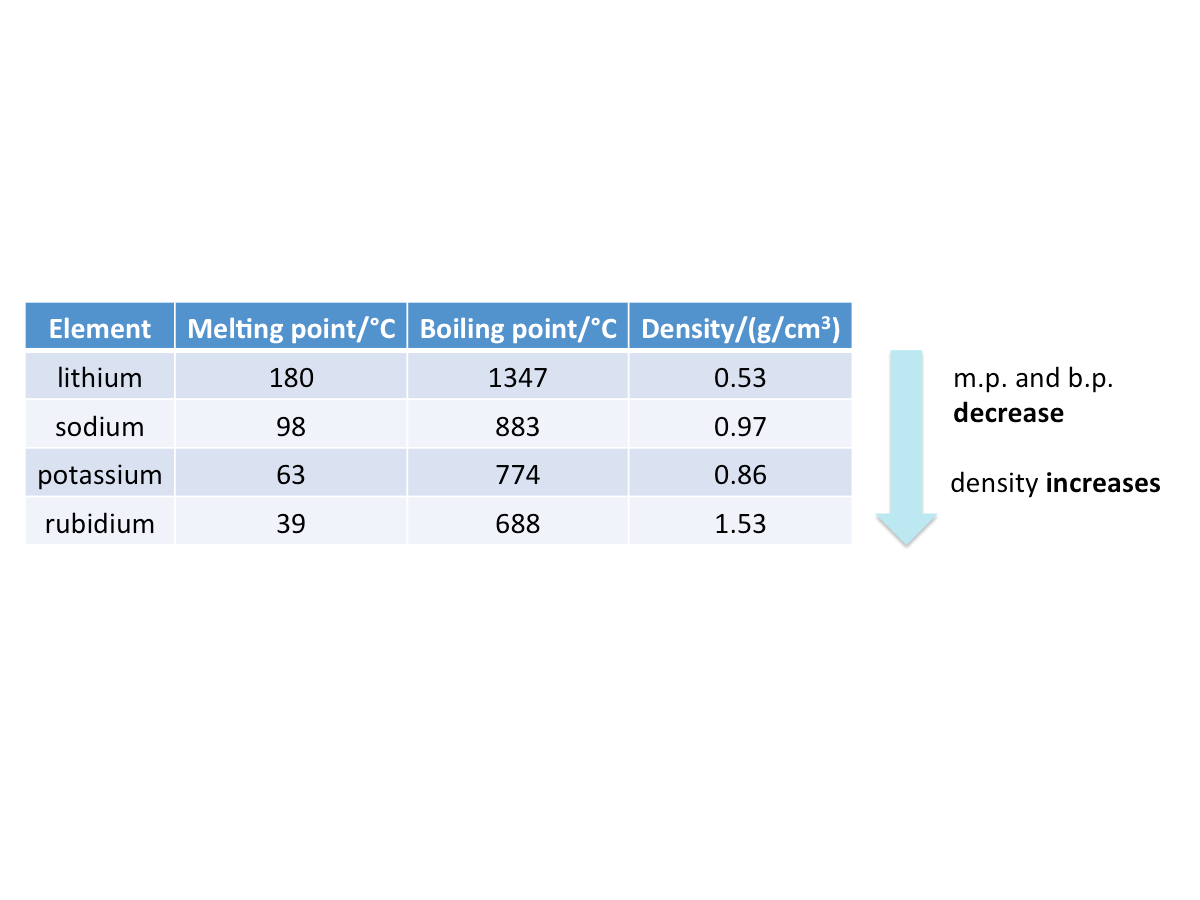
- The melting points and boiling points of alkali metals decrease
- The densities of alkali metals generally increase
The table below shows the physical properties of some group I metals.
Chemical Properties of Alkali Metals
Alkali metals
- Are highly reactive
- Are stored in oil to prevent reaction with air and water
Each alkali metal has one valence electron. By losing this valence electron, an alkali metal attains the electronic structure of a noble gas.
Going down the group,
- Size of atom increases
- Easier to lose valence electron
Hence, reactivity increases down group I.
Order of reactivity: lithium < sodium < potassium
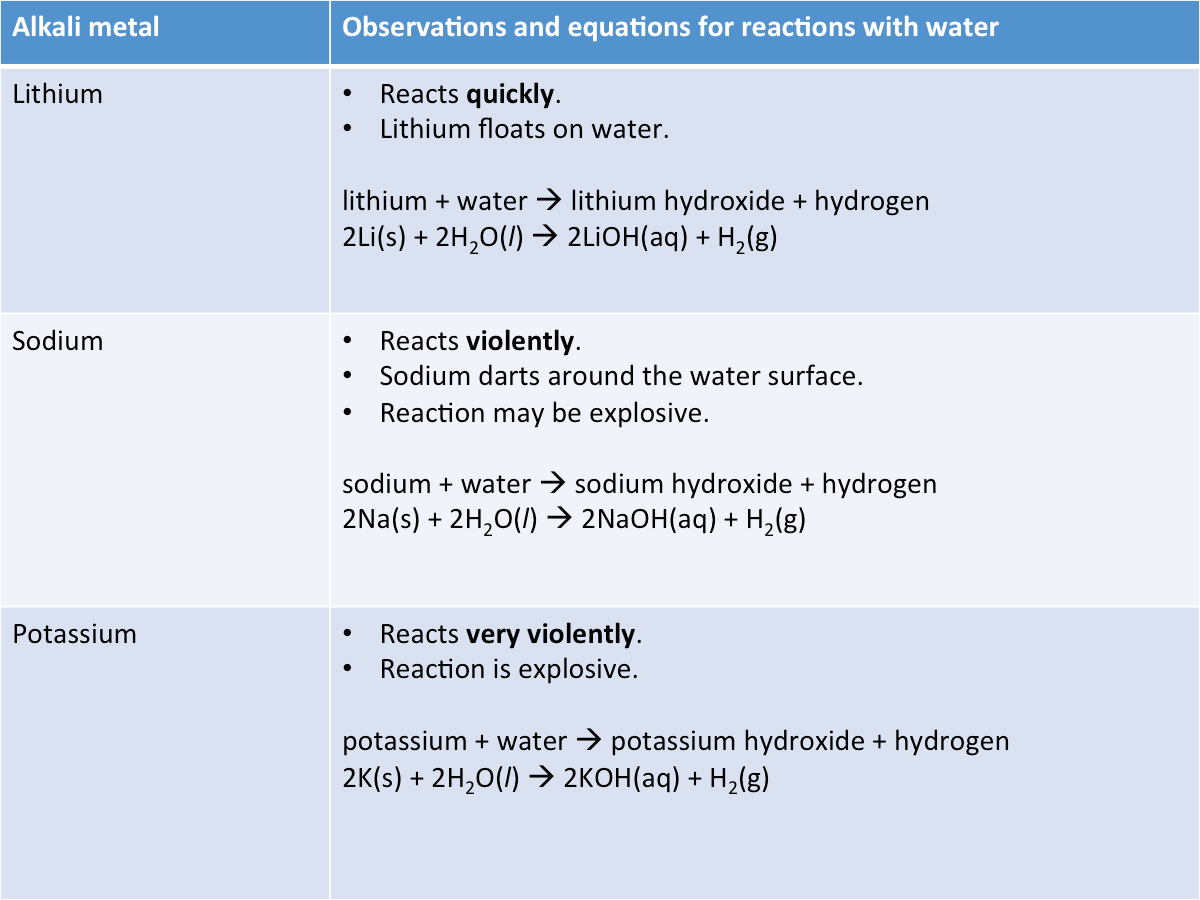
Alkali metals react with cold water to form an alkali and hydrogen.
The table below describes the reactions of some alkali metals with water.
1. Alkali metals react with cold water to form an alkali and hydrogen (video)
The video below shows reactions of alkali metals with water.
Alkali metals are powerful reducing agents
All alkalis form ion with a charge of +1 by losing one electron from the outer shell.
For example,Na --> Na+ + e-
Since the alkali metals give away their electrons readily, they behave as powerful reducing agents in all their reactions.
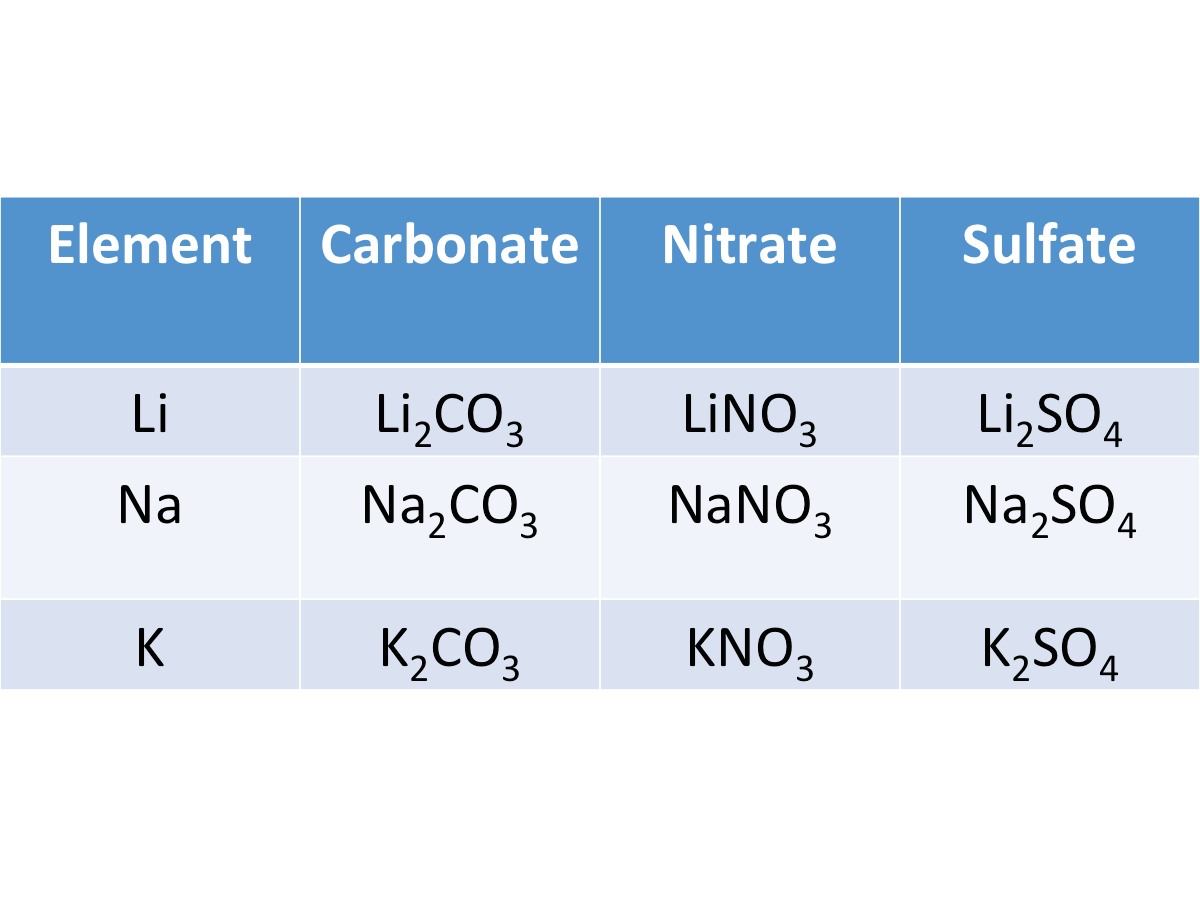
Alkali metals form ionic compounds
Their compounds have similar chemical formulae and are soluble in water.
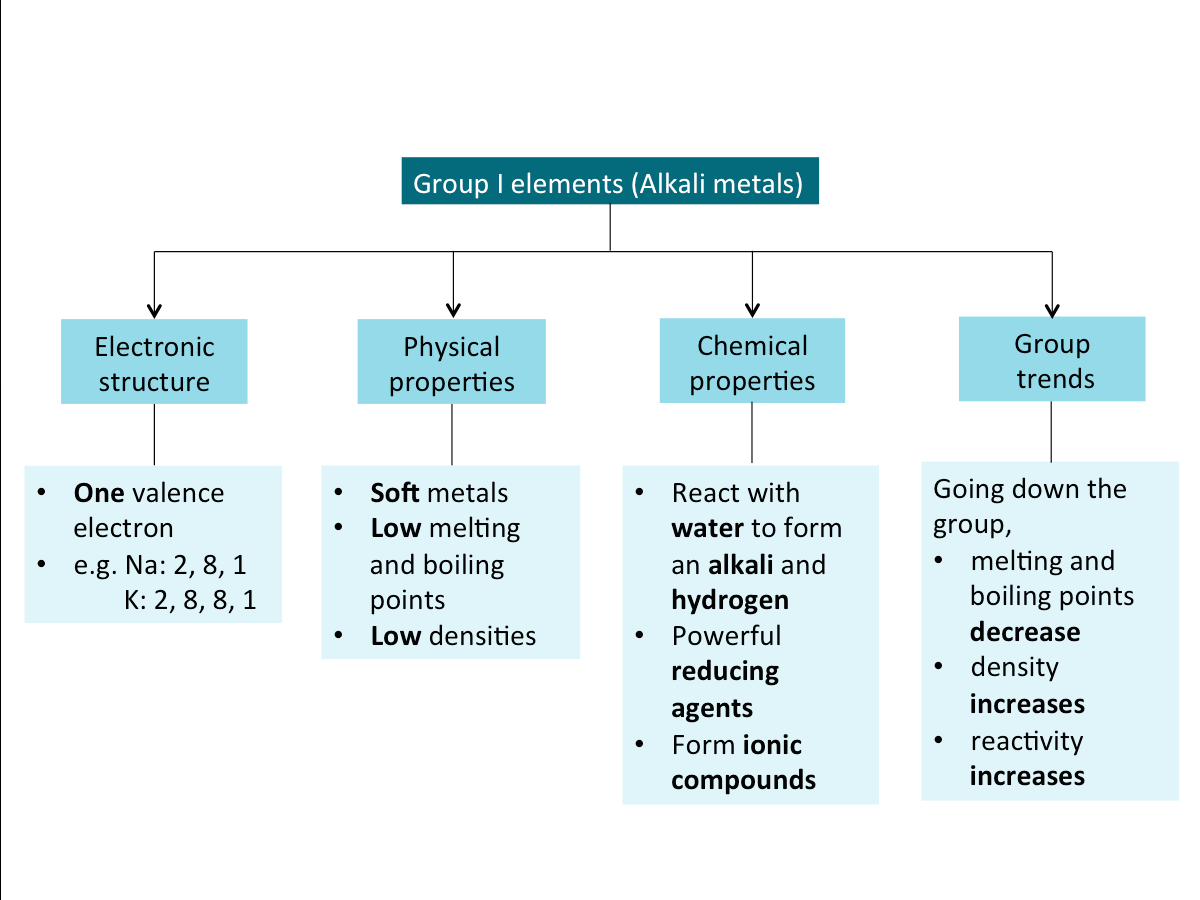
Alkali Metals (Summary)
Below is a summary of the key properties of alkali metals.
What are halogens?
Halogens are elements in Group VII of the Periodic Table.
Physical Properties of Halogens
Halogens are non-metals that exist as diatomic covalent molecules (e.g. F2, Cl2, Br2)
- They have low melting and boiling points.
- They are coloured.
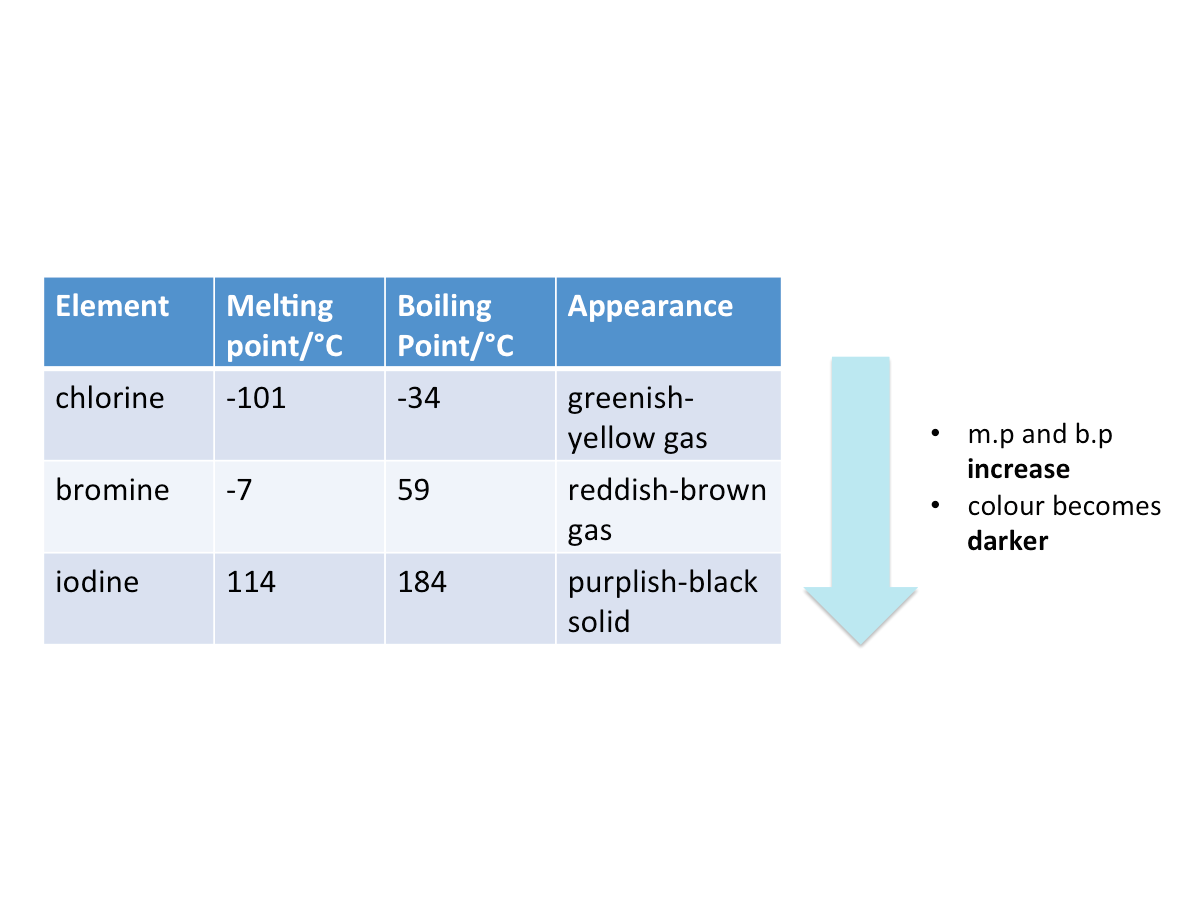
The table below shows the physical properties of some halogens.
From the table, it can be seen that going down the group,
- The melting points and boiling points increase
- Colours of halogens become darker
Chemical Properties of Halogens
Halogens are reactive non-metals. Each halogen has seven valence electrons, meaning only one more electron is needed to achieve the stable electronic configuration of a noble gas.
Halogens react with most metals to form salts called halides. Fluoride ions (F-) and chloride ions (Cl-) are examples of halide ions.
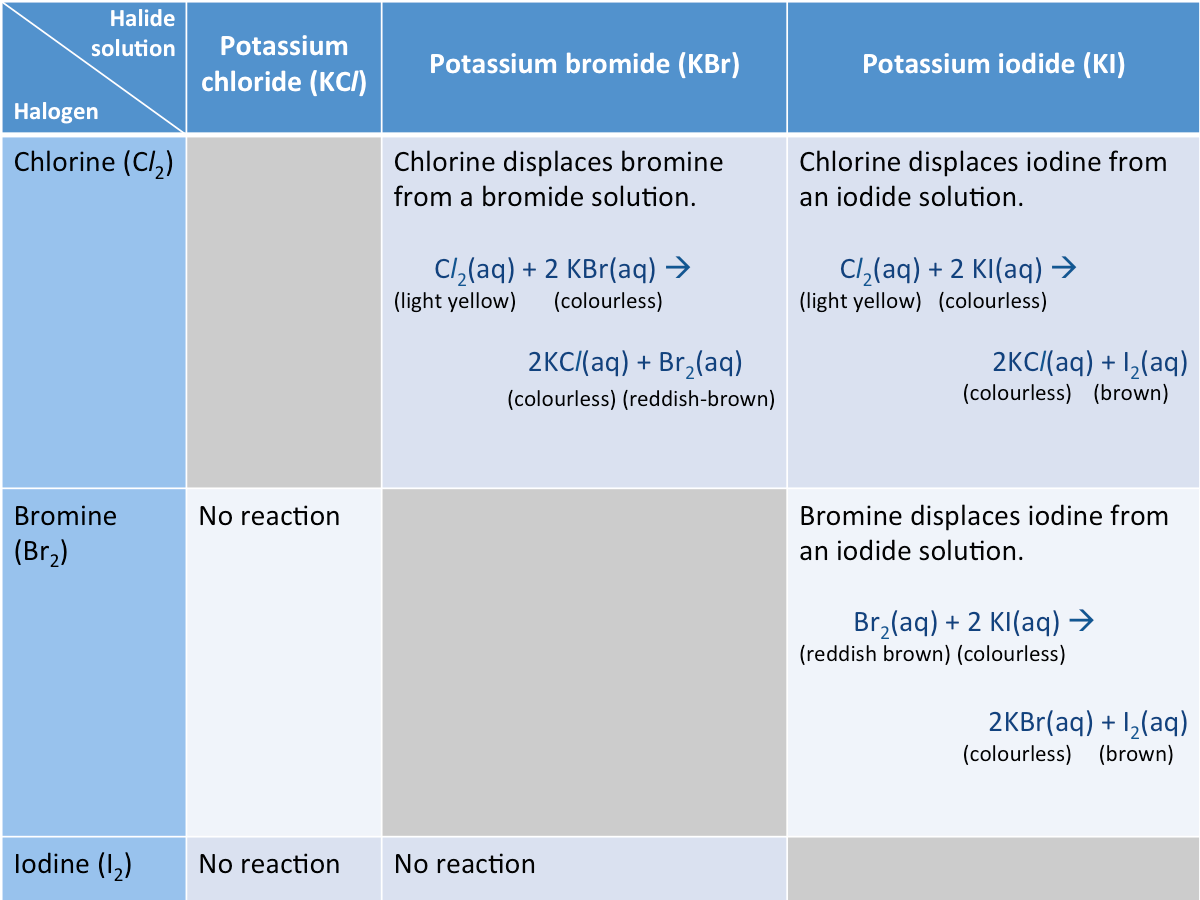
Halogens undergo displacement reactions with halide solutions
A displacement reaction is a reaction in which one element takes the place of another element in a compound.
A more reactive halogen will displace a less reactive halogen from its halide solution.
The displacement reactions of some halogens are shown in the table below. A less reactive halogen cannot displace a more reactive halogen form its halide solution.
The order of reactivity of halogens can be deduced from thier displacement reactions. Unlike alkali metals, the reactivity of halogens decreases down the group, making it more difficult for the nucleus to attract one more electron.
Order of reactivity: chlorine > bromine > iondine
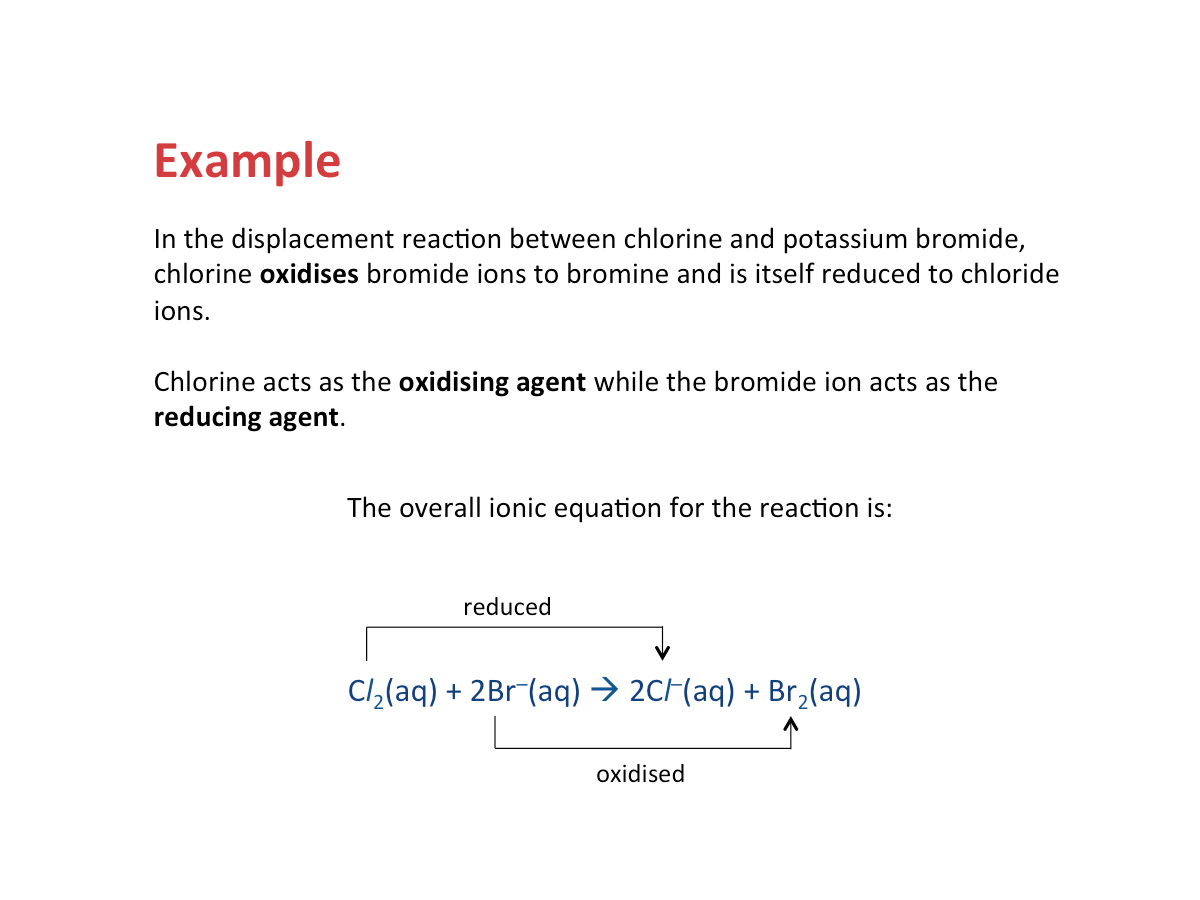
Halogens are powerful oxidising agents
During chemical reactions, atoms of halogens readily gain electrons to form halide ions with a charge of -1.
X2 + 2e- --> 2X- (where X2 = halogen, X- = halide ion)
The displacement reactions between halogens and other halide ions can also be classifed as redox reactions. An example is shown below.
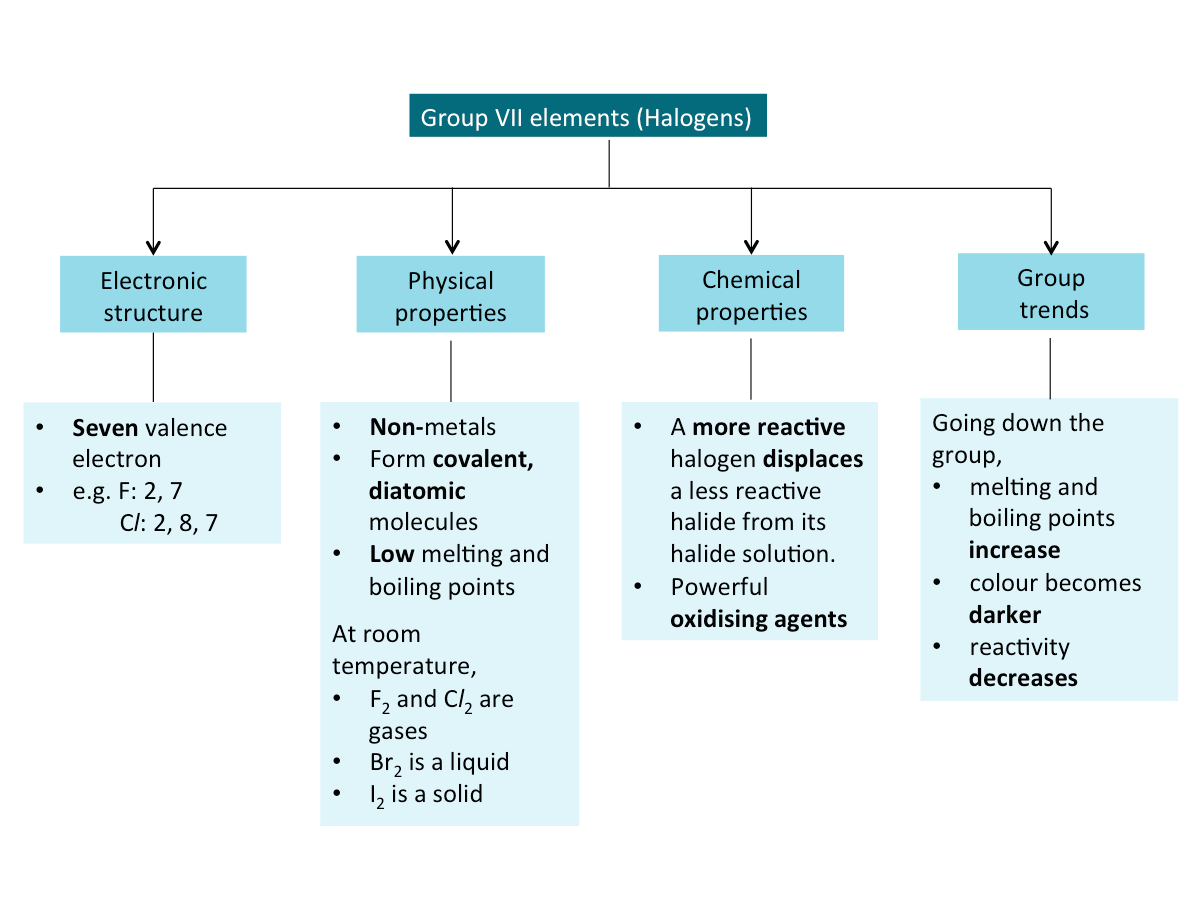
Halogens (Summary)
Below is a summary of the key properties of halogens.
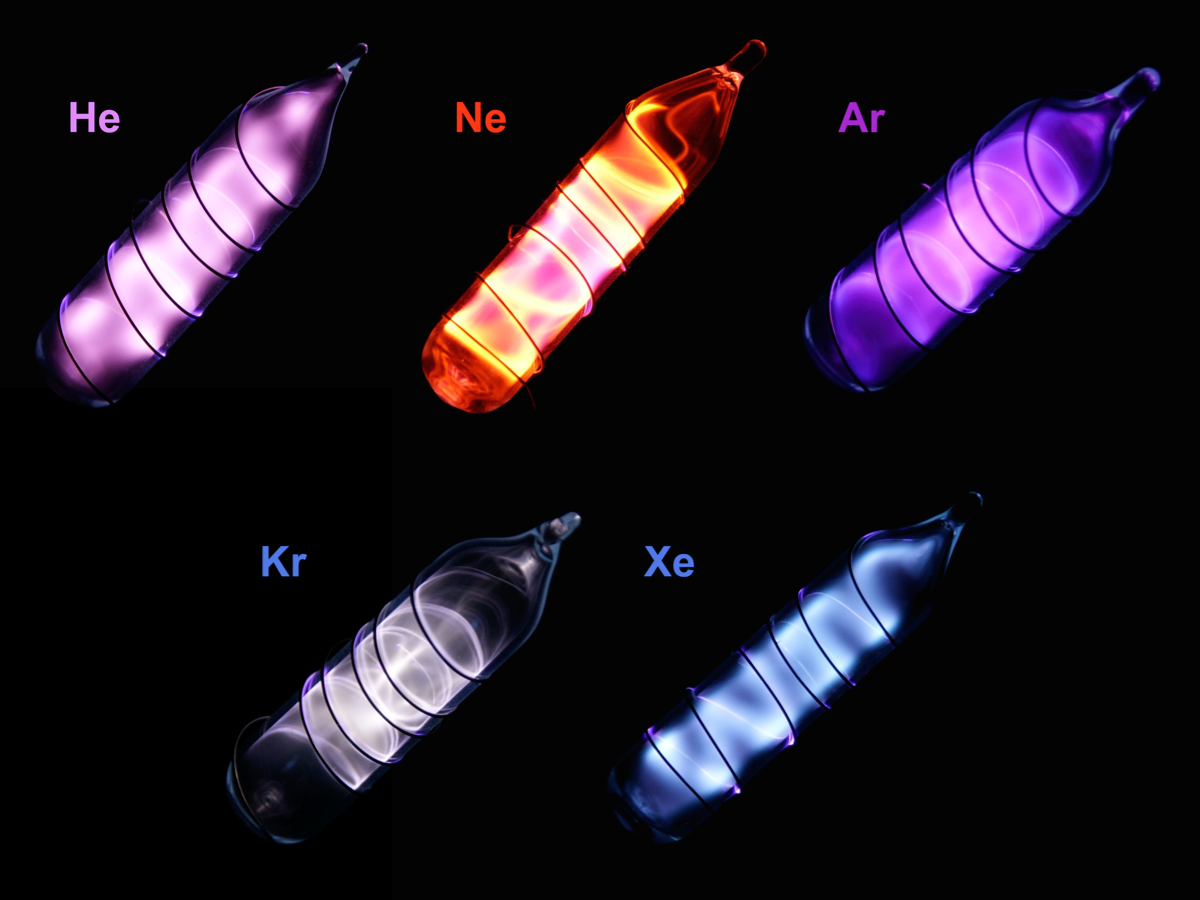
What are noble gases?
The elements in Group 0 or Group VIII are called noble gases or inert gases.
Watch the video below to learn more about noble gases.
Properties of Noble Gases
Noble gases are non-metals. They
- Are monatomic
- Are colourless gases at room temperature
- Have low melting and boiling points
- Are insoluble in water
- Are unreactive (their full electromic structures make the noble gases unreactive as they do not tend to lose, gain or share electrons. Hence, they rarely react to form compounds)
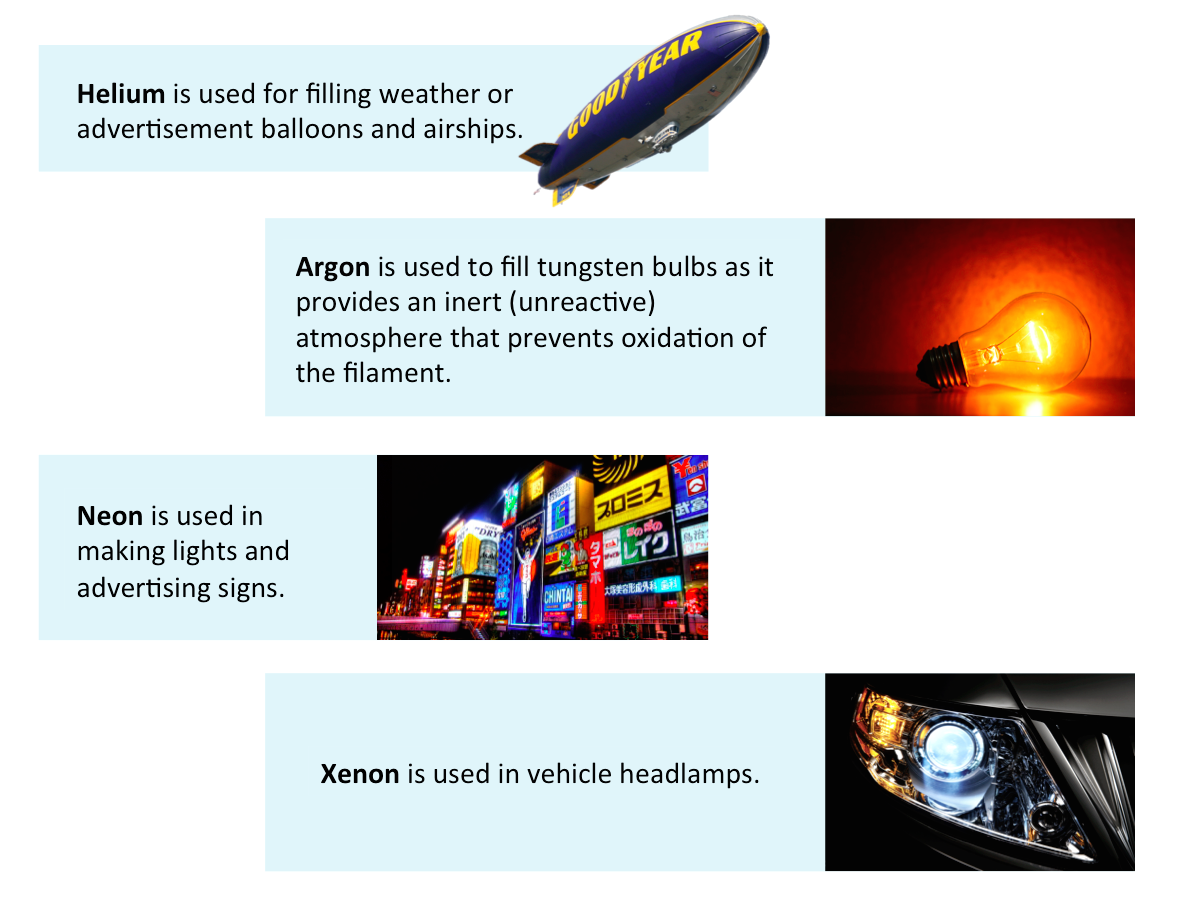
Uses of Noble Gases
Noble gases are mostly used to provide an inert atmosphere. The figure below shows some applications of noble gases.
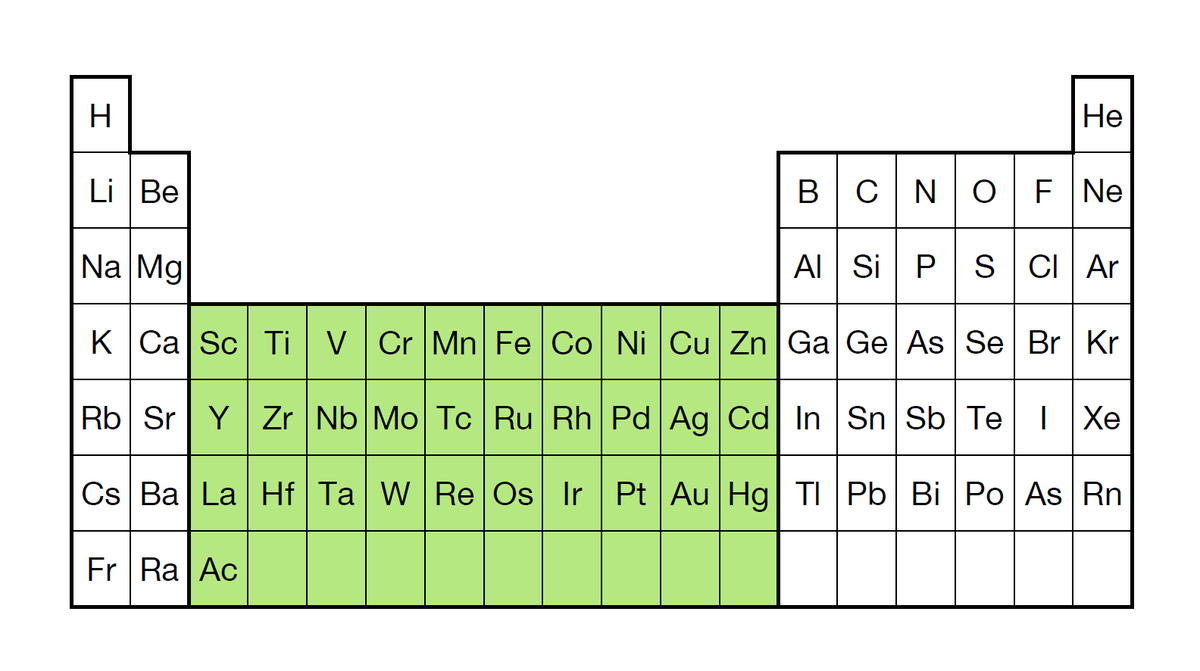
What are transition elements?
Transition elements or transition metals are the block of metals found between Groups II and III of the Periodic Table.
Properties of Transition Elements (Part 1)
1. Transition metals have high melting and boiling points and high densities.
Transition metals have much higher densities and melting and boiling points as compared to Group I and Group II metals.
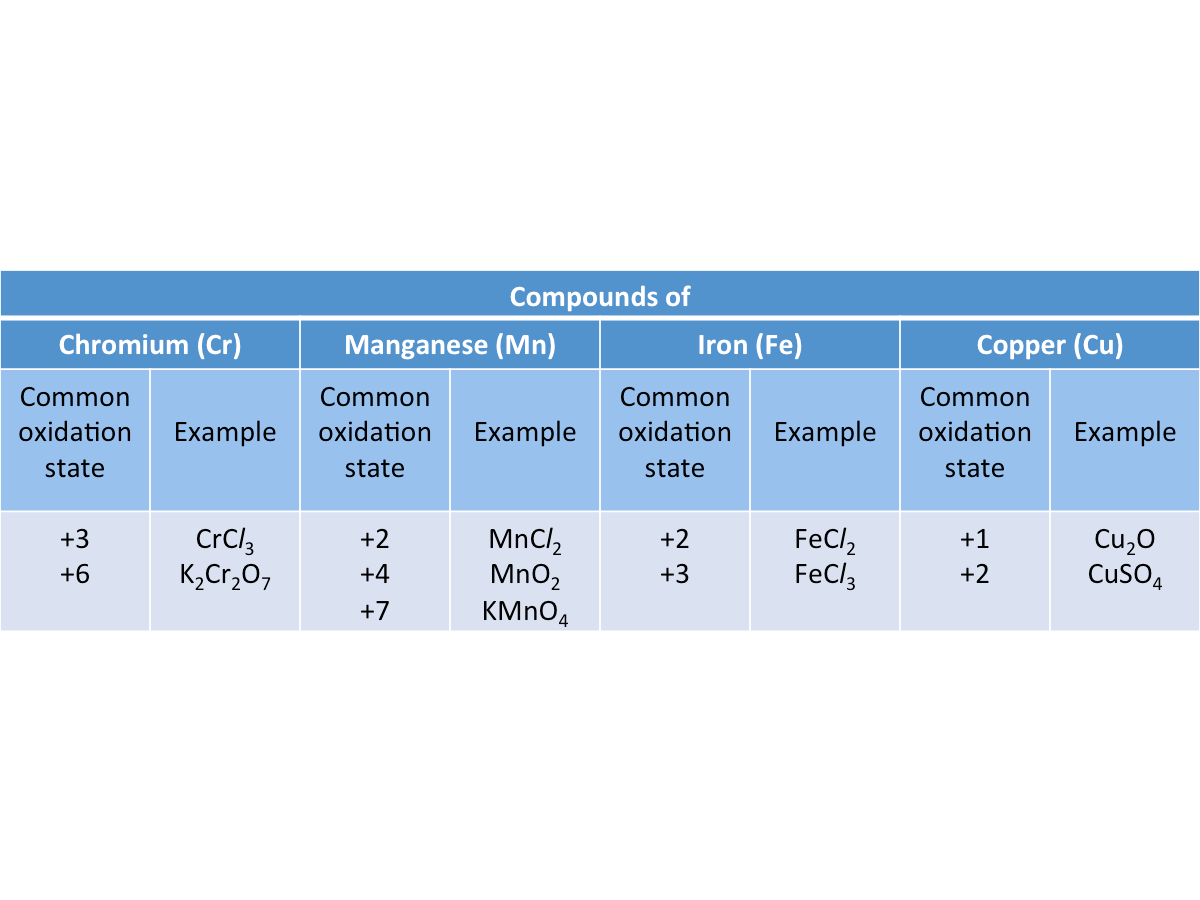
2. Transition metals have variable oxidation states.
Transition metals form ions with different oxidation states.
The table below shows the variable oxidation states of some transition metals in their compounds.
Properties of Transition Elements (Part 2)
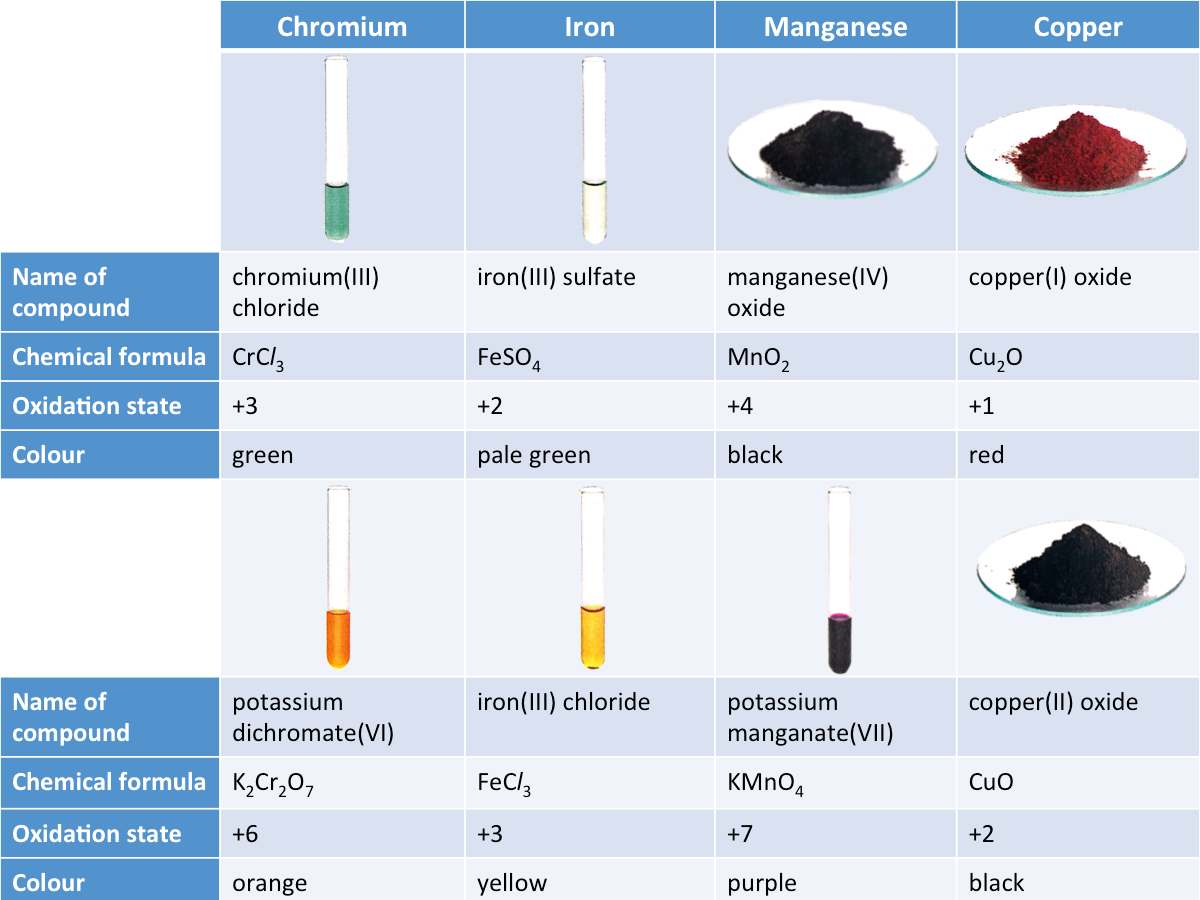
3. Transition metals form coloured compounds.
One of the most significant featues of transition metals is that they usually form coloured compounds. The colours of the compounds of a transition metal are different at different oxidation states.
The table below shows the colours and oxidation states of some transition metal compounds.
Properties of Transition Elements (Part 3)
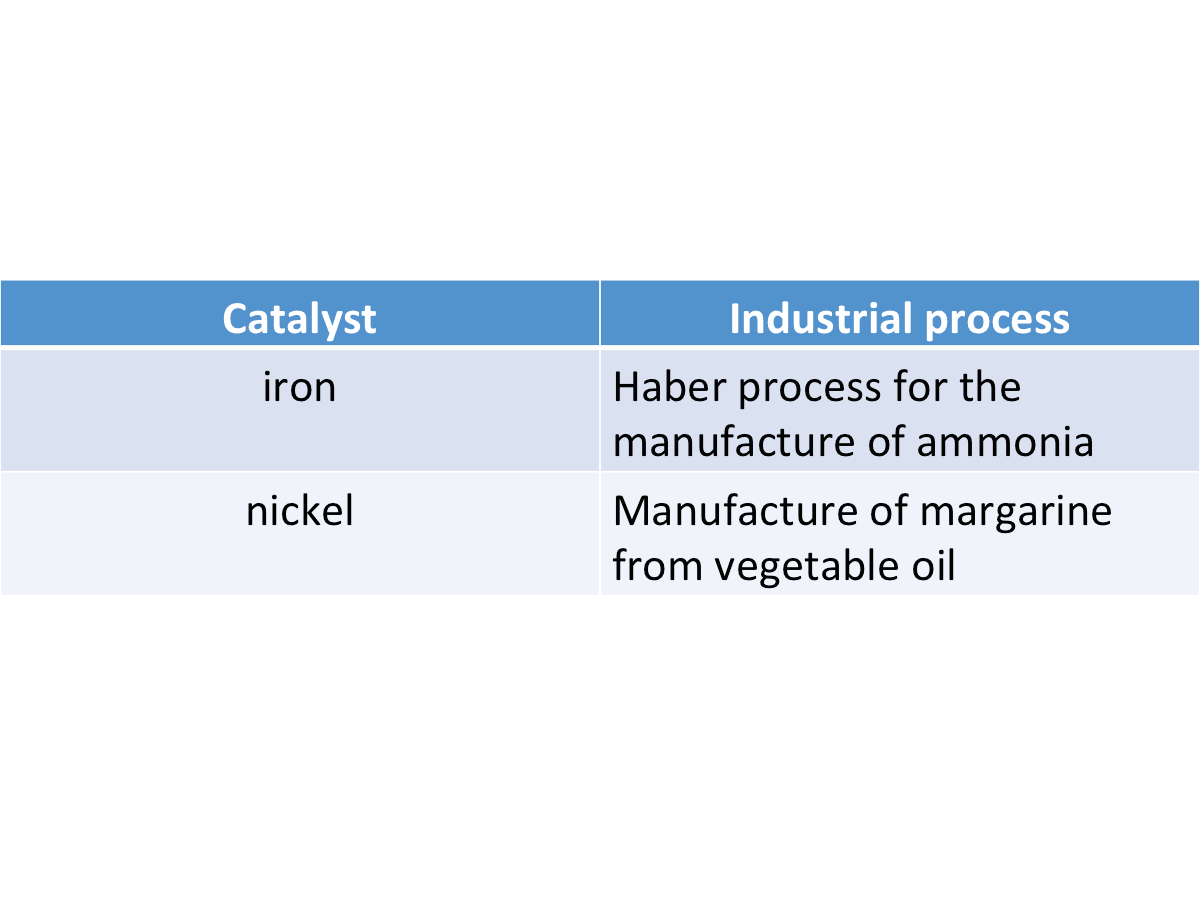
4. Transition metals and their compounds are good catalysts.
A catalyst is a substance that increases the speed of a chemical reaction and remains chemically unchanged at the end of the reaction.
The table below shows some uses of transition metals as catalysts.