Metals
Metals have the following general properties as stated in the table below. Can you come up with the reasons why they have such properties?
Why are pure metals not widely used in industry?
1. Pure metals may react with air and water and wear away or corrode easily.
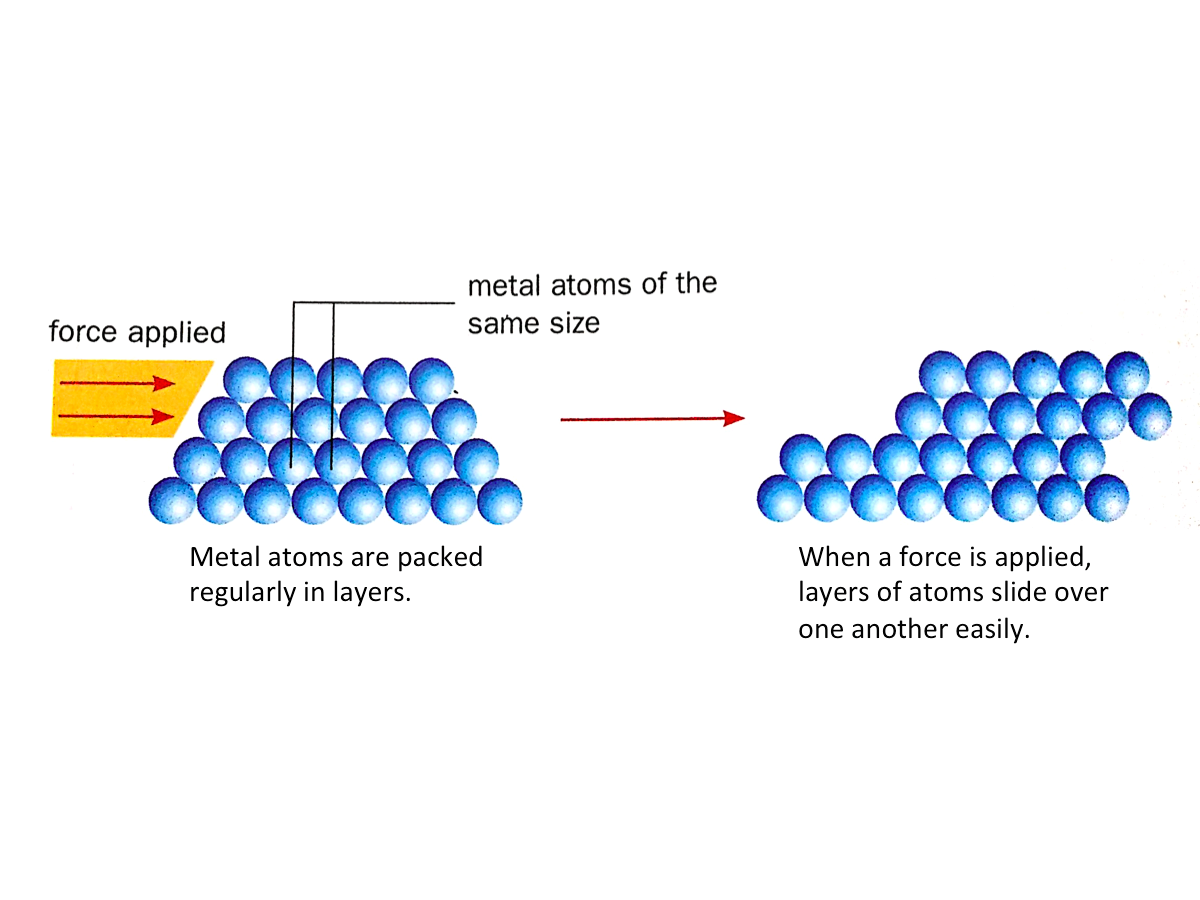
2. The atoms in pure metal are packed regularly in layers which can slide over one another easily when a force is applied. Hence, pure metals are soft.
Alloys
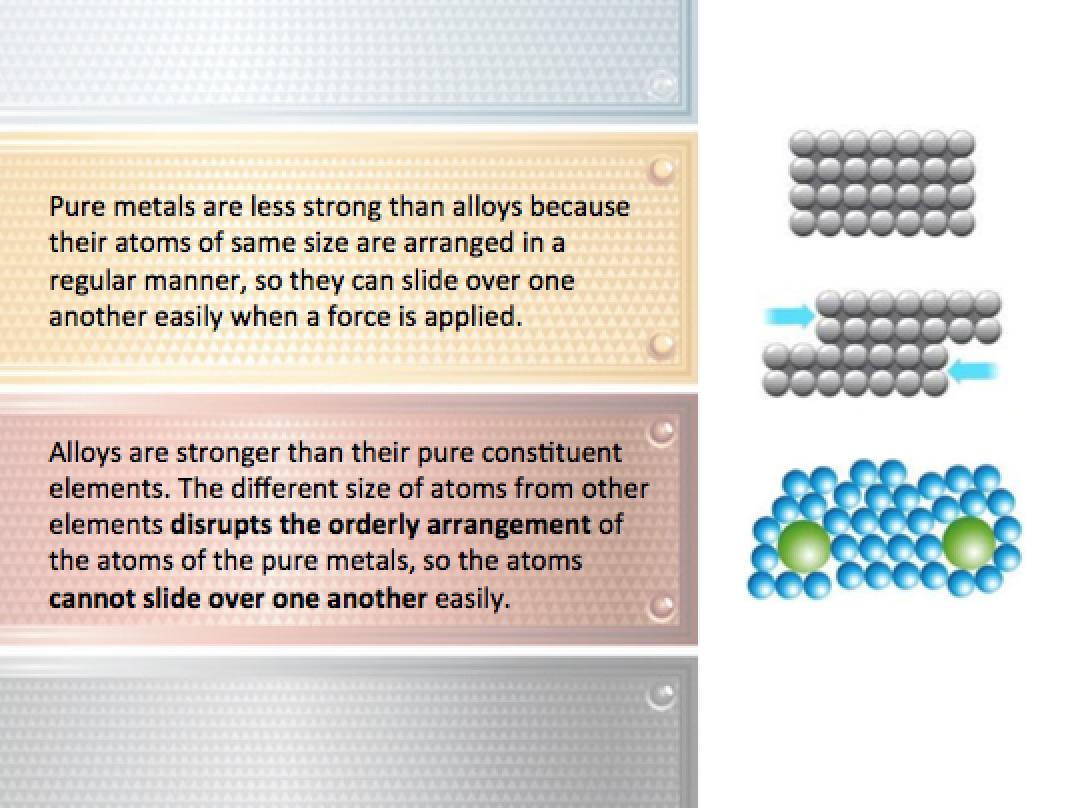
An alloy is a mixture of a metal with one or a few other elements. The physical properties of an alloy are different from their pure constitutent elements, as they are:
- Stronger and harder than pure metals, and
- More resistant to corrosion than pure metals.
Why are metals often used in the form of alloys?
Here are some uses of alloys, and use the slider to view some examples!
Determining the Order of Reactivity of Metals
What is the reactivity series?
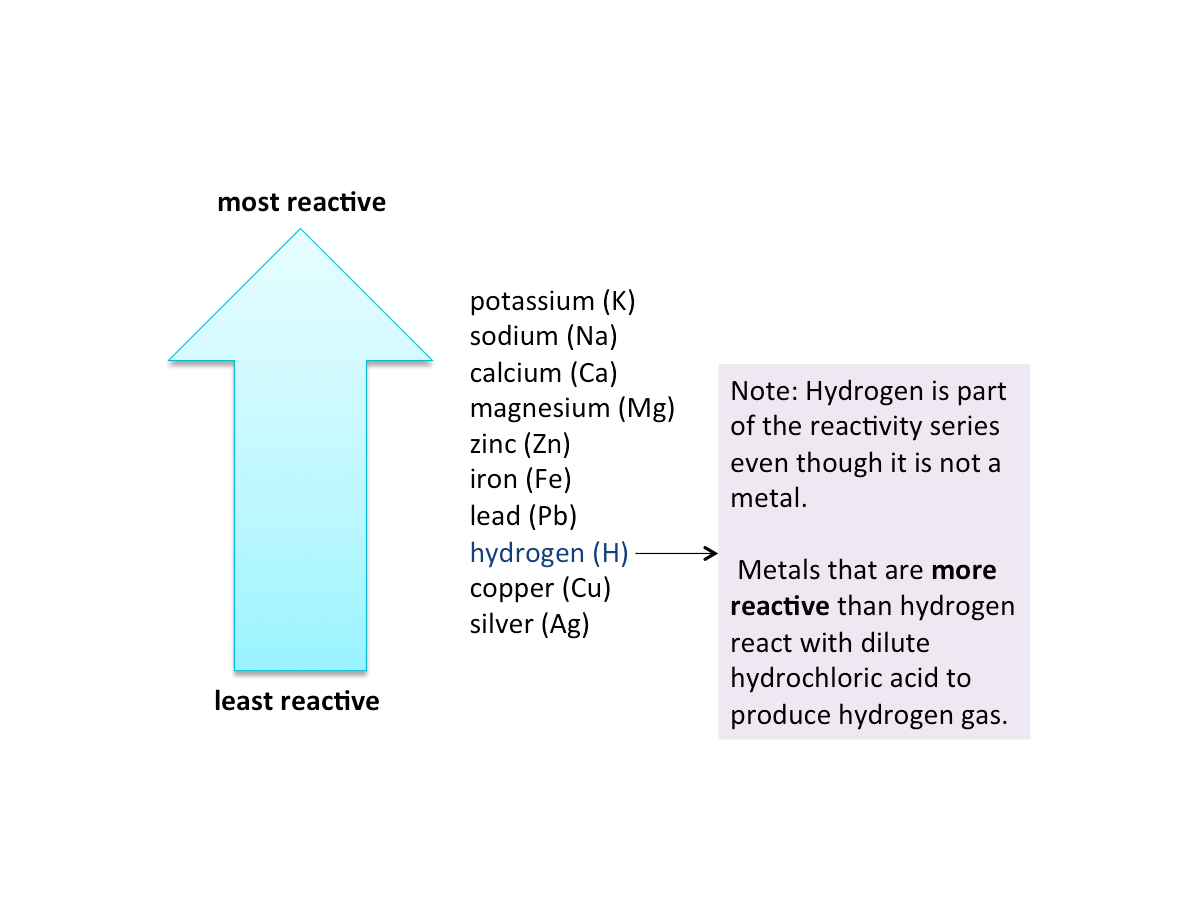
In the reactivity series, metals are aranged from the most reactive to the least reactive.
The order of reactivity of metals can be determined by observing the speed of reaction with water, steam and dilute hydrochloric acid.
Reaction of Metals with Cold Water and Steam
Some metals react with cold water/steam. A more reactive metal reacts more violently with cold water/steam.
Reaction of Metals with Water
Some metals react with cold water to form metal hydroxide and hydrogen gas. The general equation for the reaction is:
metal + water --> metal hydroxide + hydrogen
The table below shows the observations and chemical equations for the reactions of some metals with cold water.
Reaction of Metals with Steam
Some metals react with steam to form metal oxide and hydrogen gas. Zinc and iron do not react with cold water but they do react with steam. The general equation for the reaction is:
metal + steam --> metal oxide + hydrogen
The table below shows the observations and chemical equations for the reactions of some metals with steam.
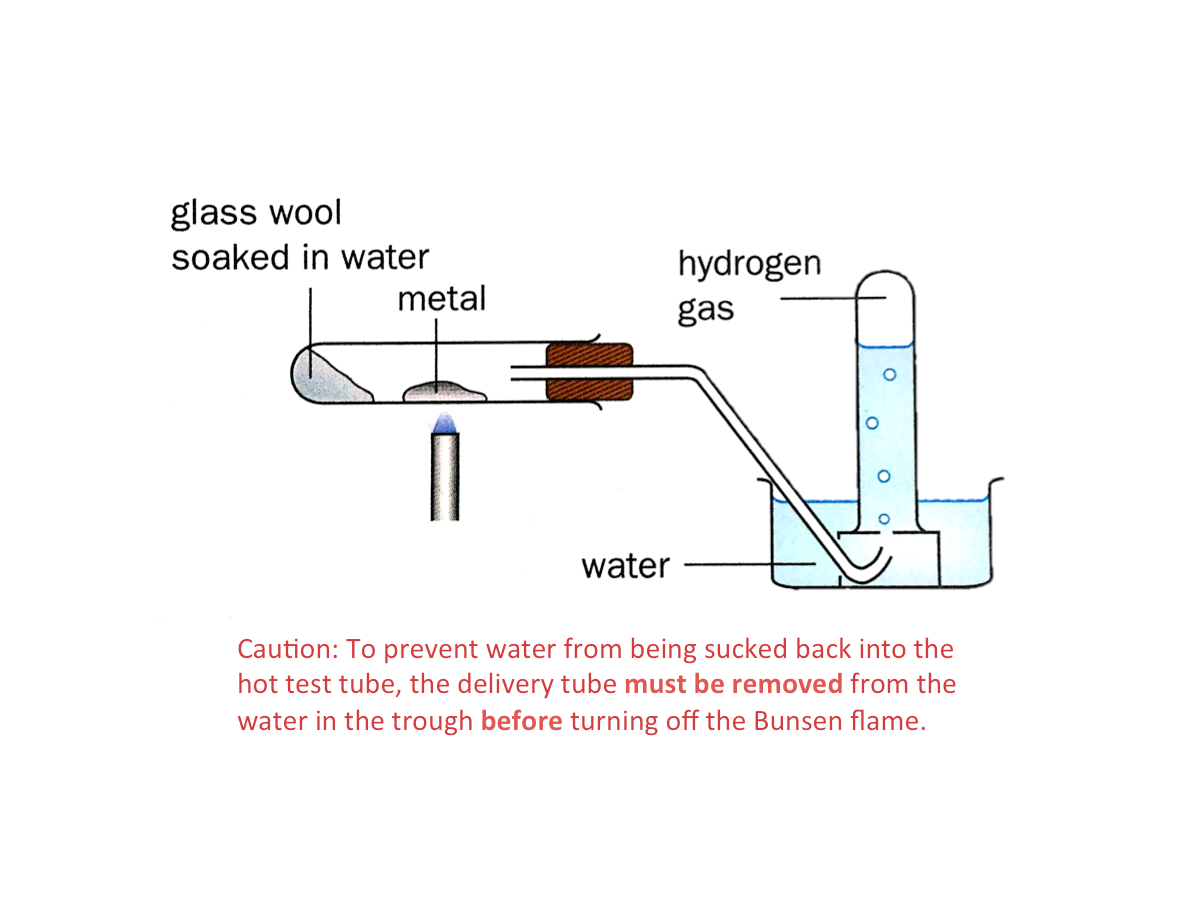
Reaction of Metals with Dilute Hydrochloric Acid
When a metal reacts with dilute hydrochloric acid, the products are metal chloride and hydrogen gas. The general equation for the reaction is:
metal + dilute hydrochloric acid --> metal chloride + hydrogen
A more reactive metal reacts more violently.
The table below summarises the reactions of some metals with dilute hydrochloric acid.
The Reactivity Series
Based on the reactions of metals with cold water, steam and dilute hydrochloric acid, the metals can be placed in order of their reactivity.
Remembering the Reactivity Series
Watch the video below to help you remember the reactivity series.
Relating the Reactivity of Metals to Their Tendency to Form Positive Ions
The reactivity of a metal is related to its tendency to form positive ions.
A more reactive metal has a greater tendency to form positive ions compared to a less reactive metal.
The reactivity series may be used to explain the following reactions:
- Displacement reactions of metals
- Reaction between a metal and the oxide of another metal
- Reduction of metal oxides with carbon
- Reduction of metal oxides with hydrogen
- Action of heat on metal carbonates
Displacement Reactions of Metals
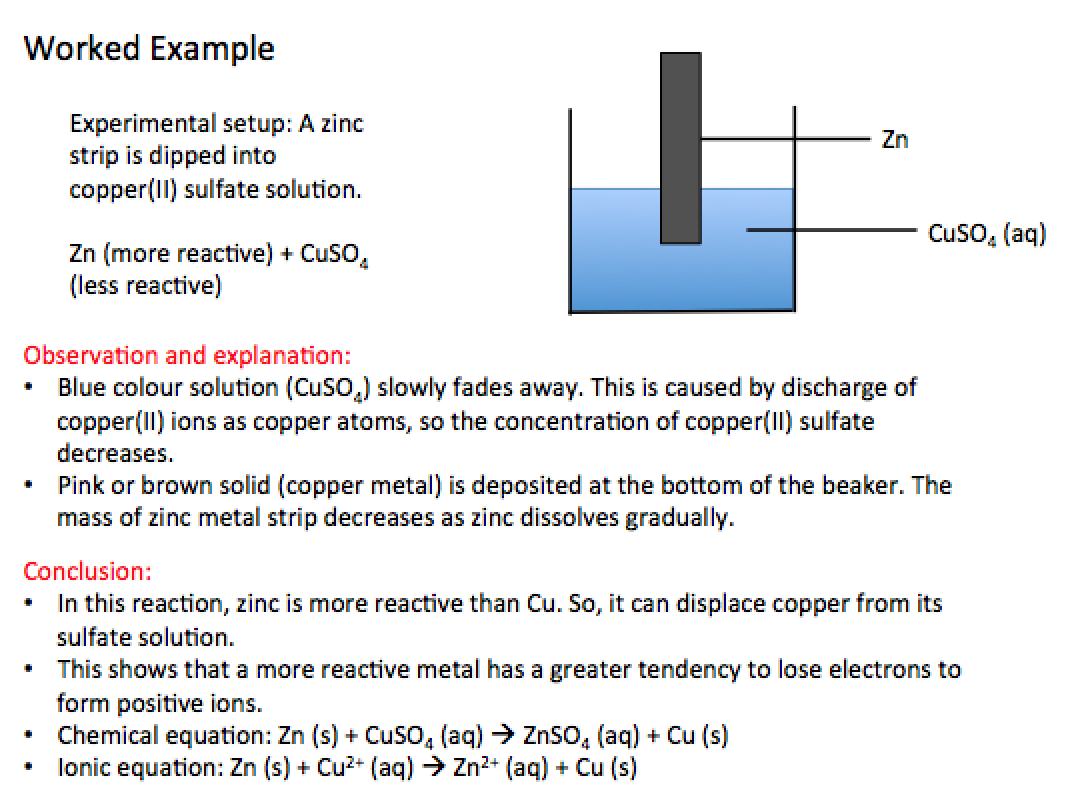
A more reactive metal can displace a less reactive metal from its salt solution.
In a displacement reaction,
- The atoms of a more reactive metal tend to lose electrons to form positive ions. The positive ions will enter the solution.
- The ions of a less reactive metal in the solution will gain electrons and are discharged as atoms of an element.
When the displacement of metal takes place, the following changes could be observed:
- Deposit of a solid metal
- Change in colour of solution
Below is an example of a displacement reaction involving salt solutions, including the expected observations, and equations to be written.
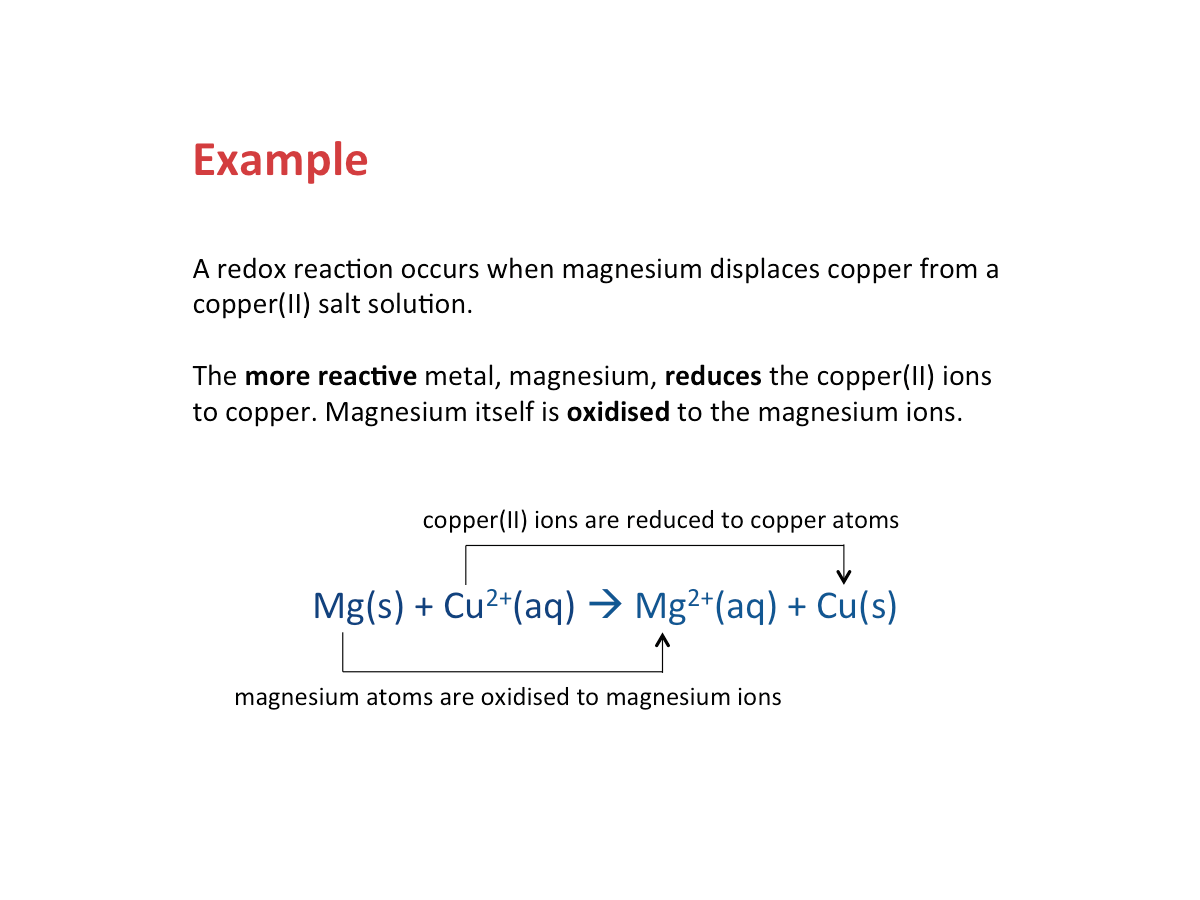
Are metal displacement reactions redox reactions?
In a metal displacement reaction, the more reactive metal is oxidised, while the less reactive metal is reduced. Thus, a displacement reaction is a redox reaction.
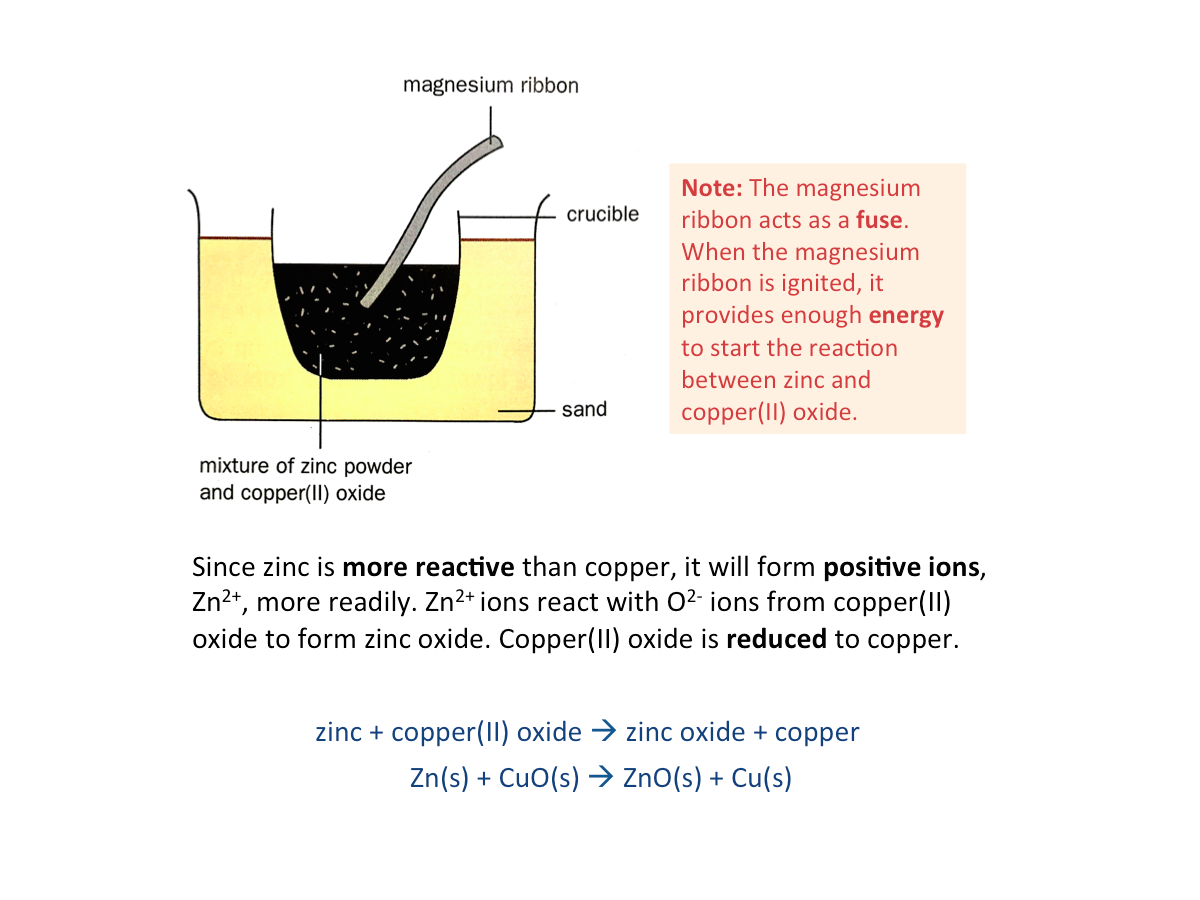
Reaction Between a Metal and the Oxide of Another Metal
A more reactive metal can reduce the oxide of a less reactive metal.
More reactive metals become ions more readily and form compounds. On the other hand, unreactive metals tend to stay uncombined. The example below shows the reaction between zinc and copper(II) oxide.
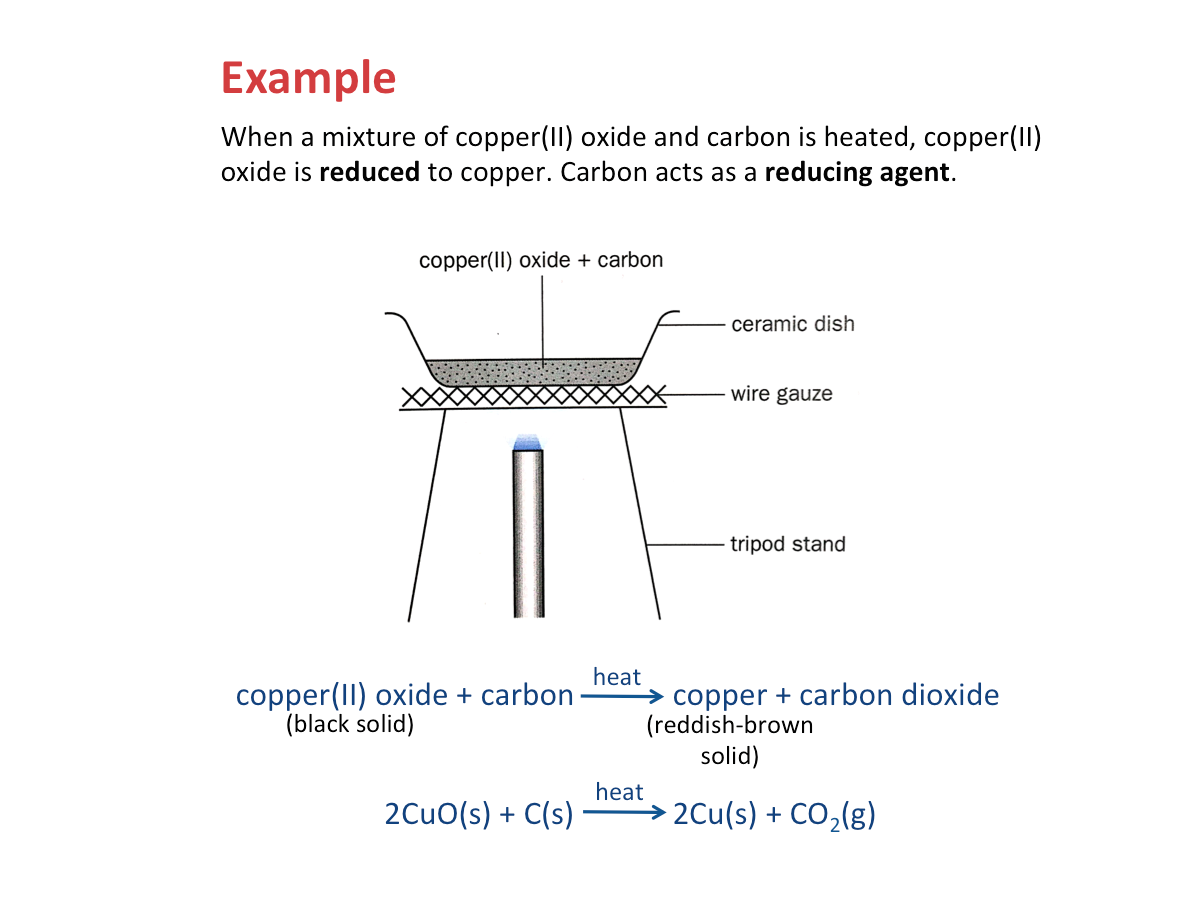
Reduction of Metal Oxides with Carbon
The general equation for the reduction of metal oxides by heating with carbon is:
metal oxide + carbon (heat) --> metal + carbon dioxide
The more reactive a metal is, the more difficult it is to reduce its oxide to the metal by carbon.
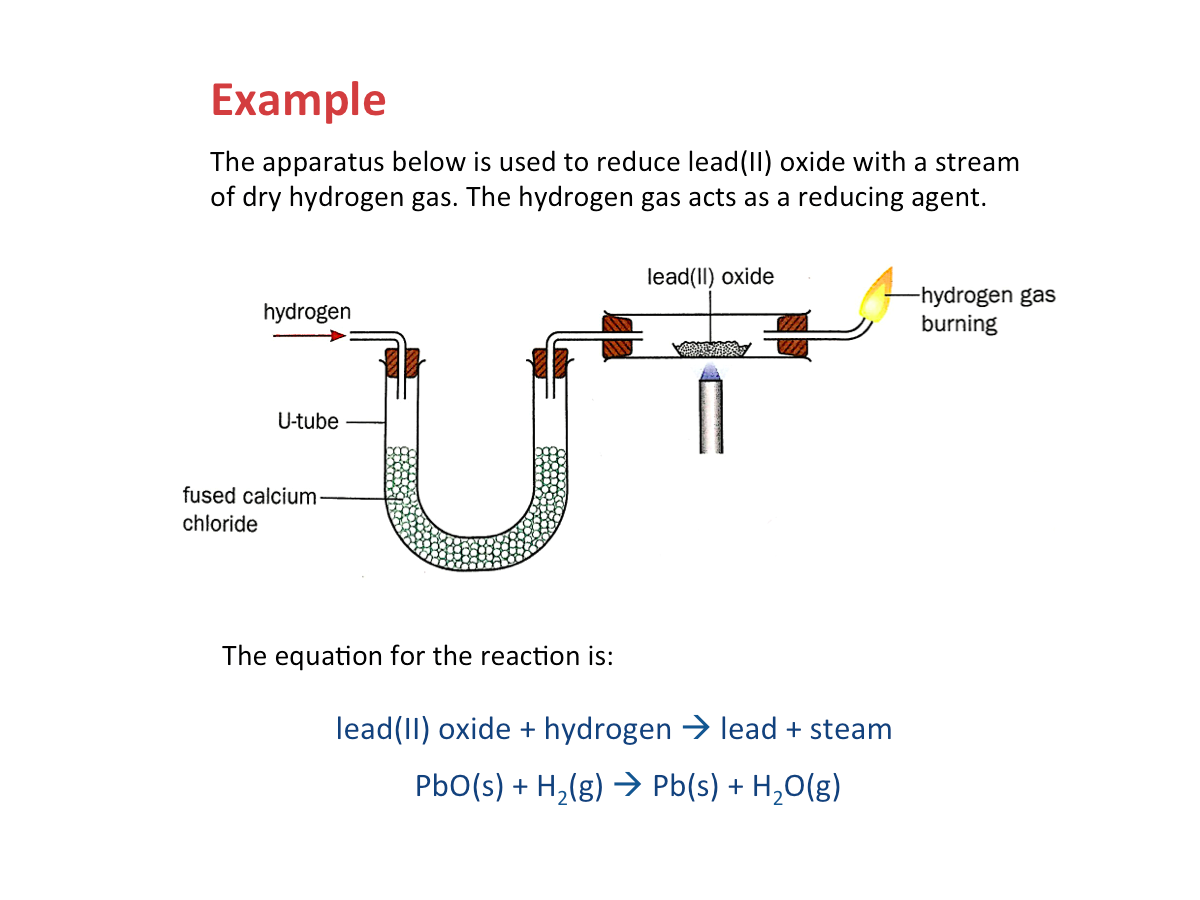
Reduction of Metal Oxides with Hydrogen
Hydrogen can also be used to reduce the oxides of some metal oxides to their metals. The general equation for the reaction is:
metal oxide + hydrogen --> metal + steam
The more reactive a metal is, the more difficult it is to reduce its oxide to the metal by hydrogen.
The oxides of reactive metals (e.g. potassium, sodium, calcium, magnesium and zinc), are often not reduced by hydrogen.
Reduction of Metal Oxides with Carbon and Hydrogen (Video)
The video below shows how metal oxides can be reduced with carbon and hydrogen.
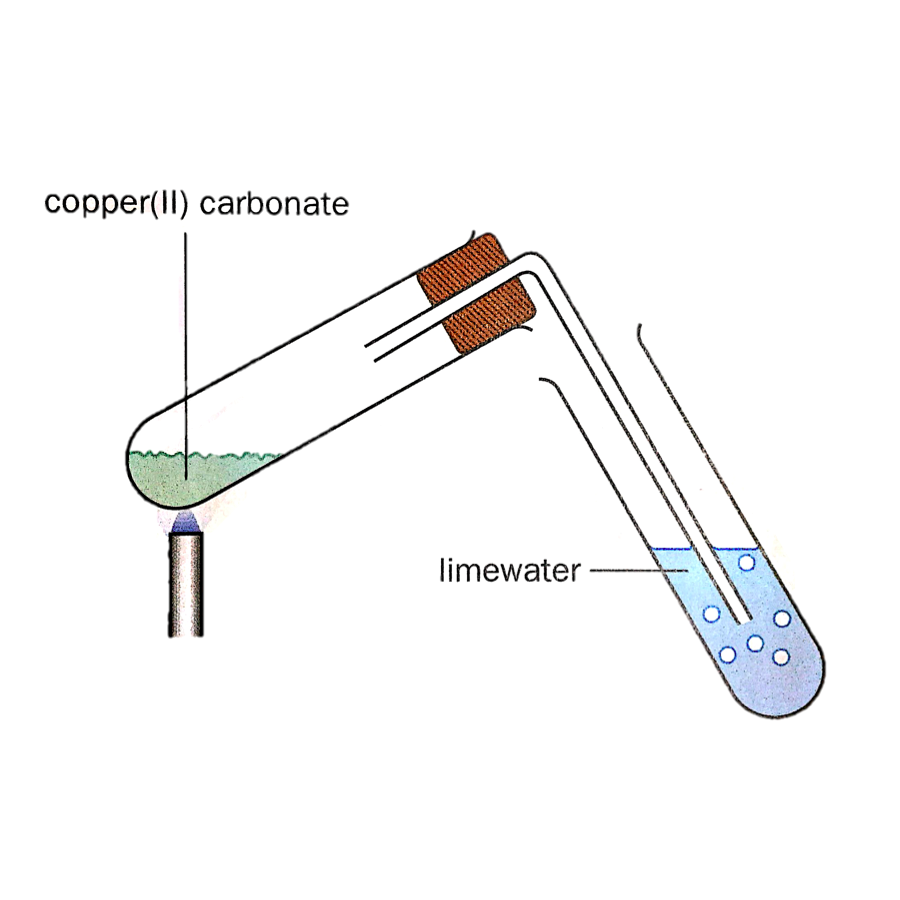
Action of Heat on Metal Carbonates
A carbonate compound decomposed upon strong heating produces an oxide and carbon dioxide.
General word equation: metal carbonate (s) --> metal oxide (s) + carbon dioxide (g)
E.g. decomposition of calcium carbonate upon strong heating gives:
CaCO3 (s) --> CaO (s) + CO2 (g)
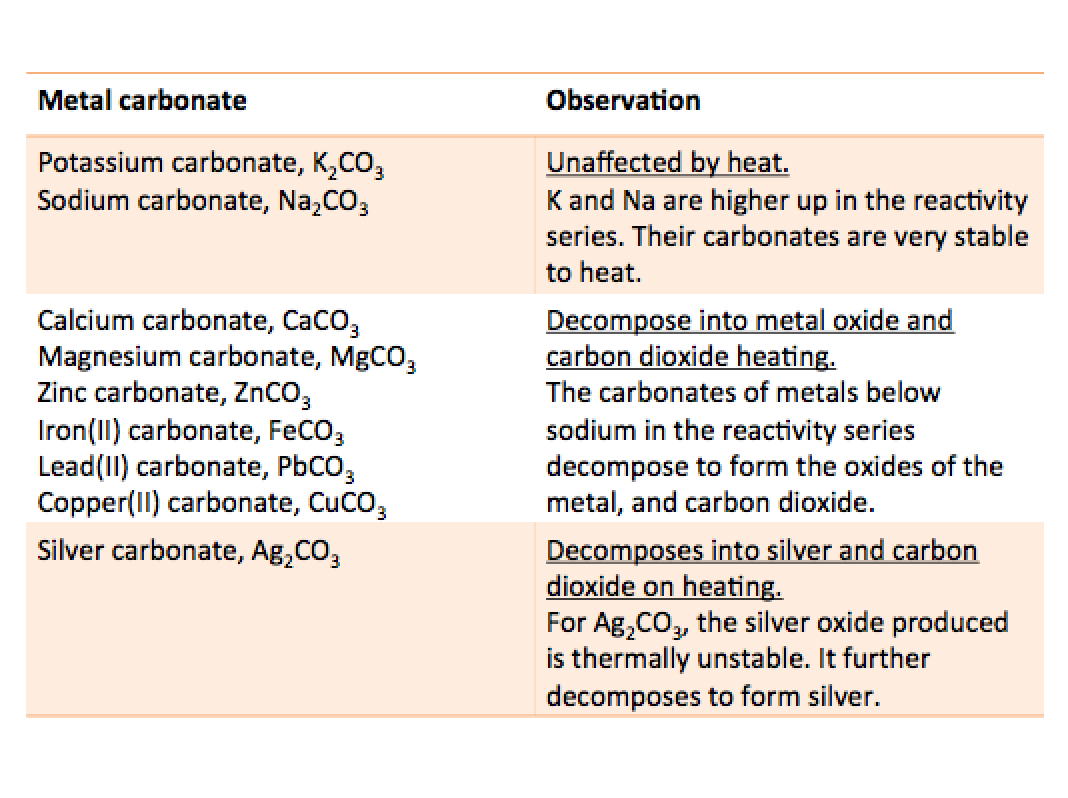
The more reactive a metal is, the more difficult it is to decompose its carbonate by heat, meaning it is more heat stable. Carbonate compounds containing group I metals are generally very heat-stable.
E.g. upon heating samples of Na2CO3 and CaCO3, CaCO3 decomposes first but Na2CO3 remains unchanged. Since sodium is more reactive than calcium, Na2CO3 is more stable than CaCO3 which has greater resistance to decompose.
The thermal stability of metal carbonates can be tested by heating them in a dry test tube as shown below. If the compound has decompos
Thermal Stability of Metal Carbonates
The table below shows the thermal stablility of some metal carbonates.
To deduce the order of reactivity of metals
The reactivity series can be used to:
- Predict the behavior of a metal from its position in the reactivity series;
- Predict the position of an unfamiliar metal in the reactivity series from a given set of experimental results.
Try out the sample questions below to get a better understanding of how to solve such questions!
Methods of Extracting Metals from Their Ores
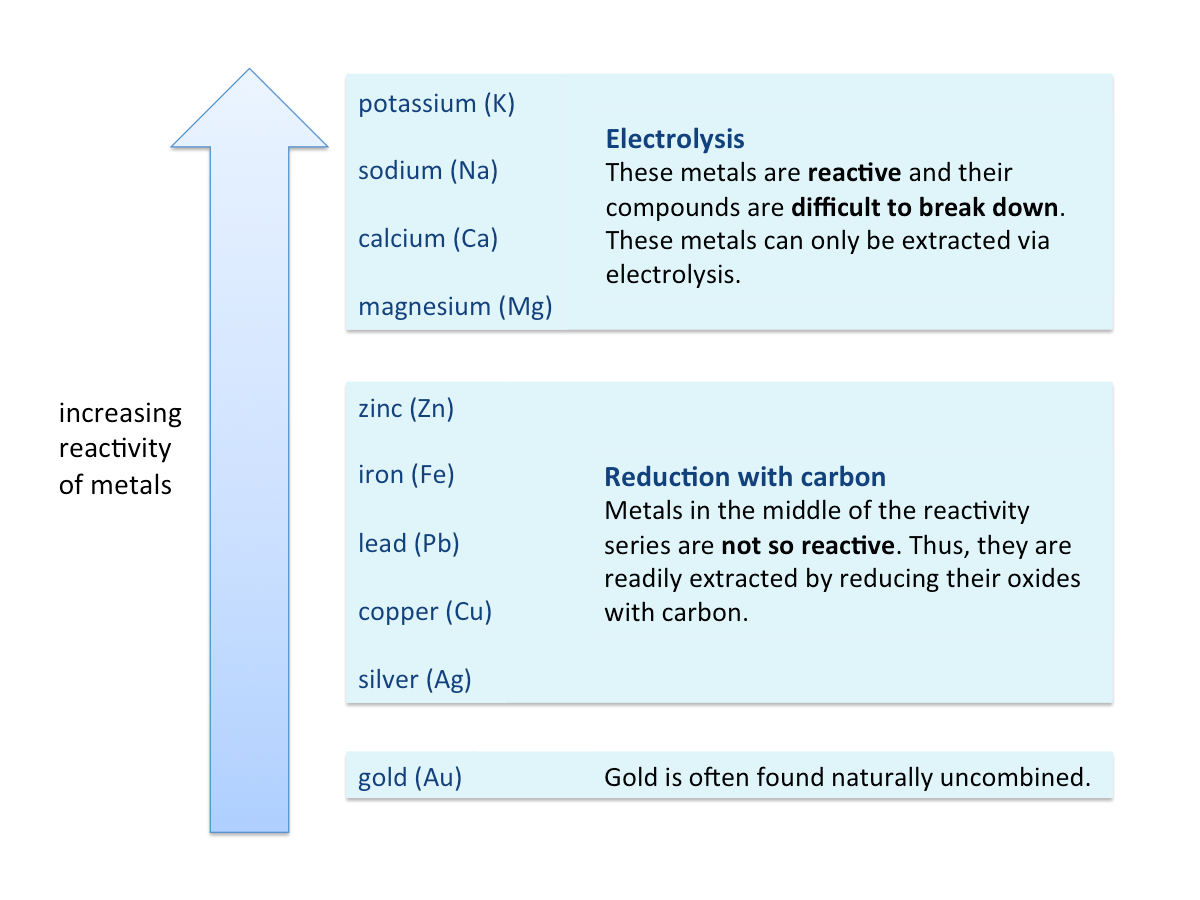
There are two main methods for extracting metals from their ores:
1. Reducing the metal compound (ore) to the metal using carbon
2. Electrolysis - using electricity to decompose the molten metal compound (ore) to the metal
The position of a metal in the reactivity series determines the method used for extraction. The more reactive the metal is, the harder it is to extract the metal from its ore.
The methods used for extracting some common metals are summarised in the figure below.
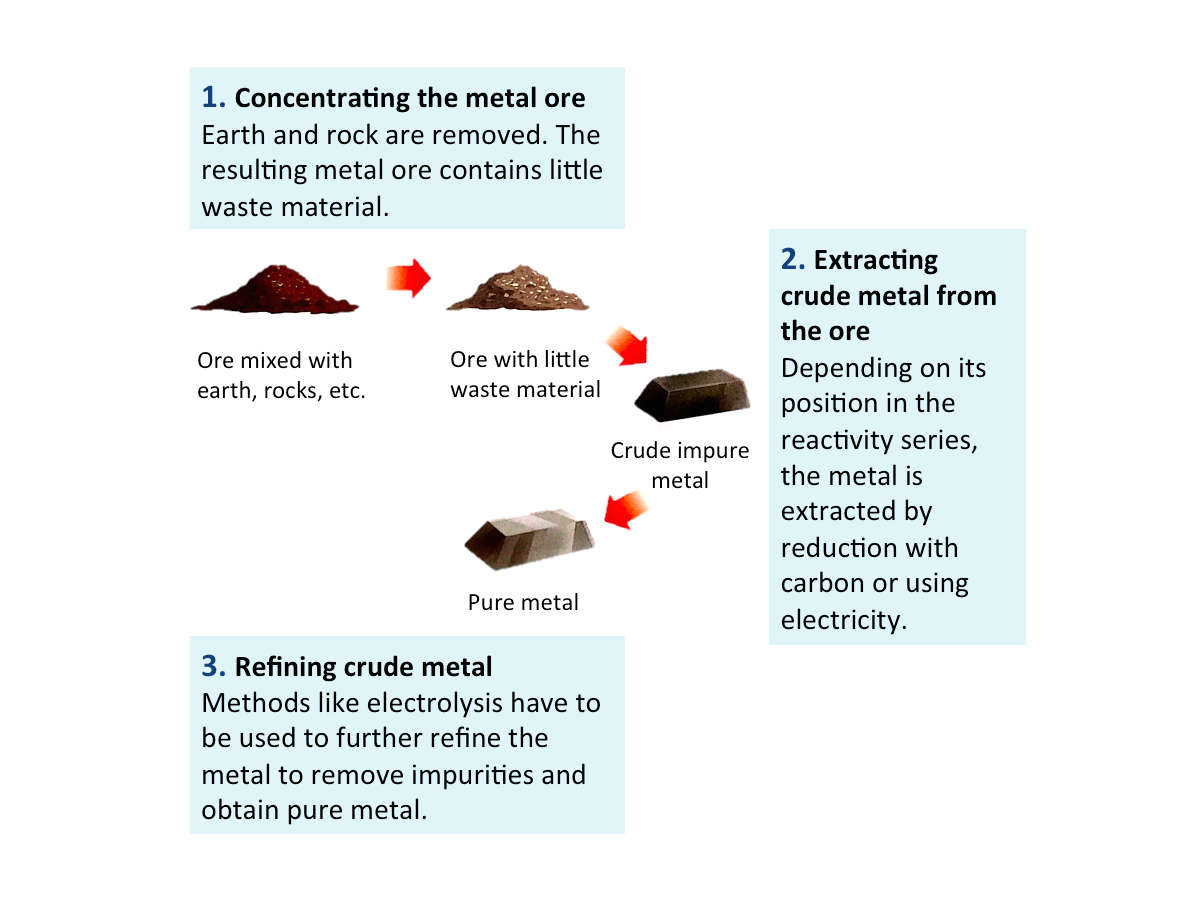
Main Stages Invoved in Extracting Metals
Only a few metals (the very unreactive ones), such as gold and platinum, occur freely in nature as uncombined metals. Most metals occur in the form of ores. An ore is a compound of the metal (usually the oxides, sulfides, chlorides or carbonates) mixed with large amounts of earth and rock.
The figure below shows the three mains stages involved in obtaining metals from their ores.
Extraction of Metals (Video)
Watch the video below for a better understanding of how metals are extracted.
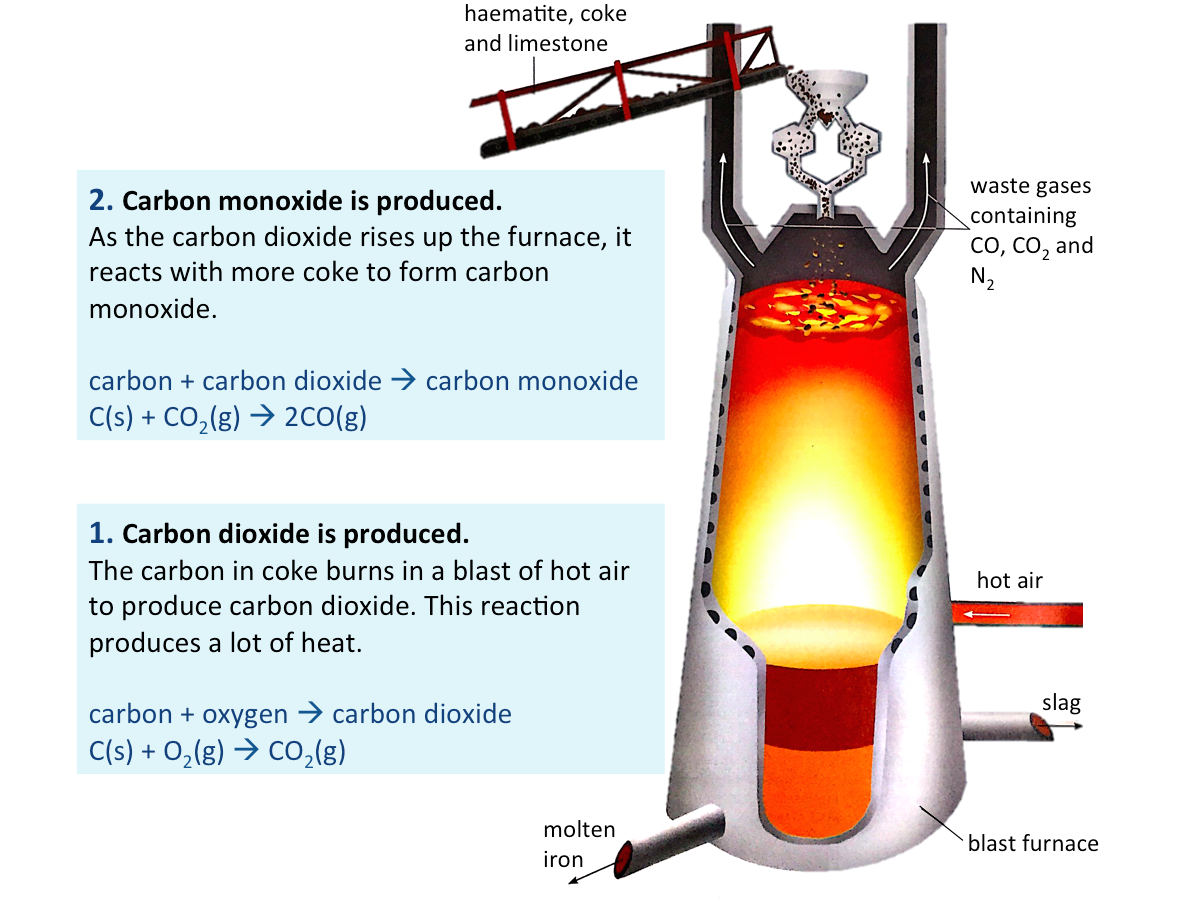
Extracting Iron from Haematite
The main ore of iron is haematite. Haematite contains iron(III) oxide mixed with impurities such as sand and clay. Iron is extracted from haematite in a blast furnace.
Haematite, coke (which is mainly carbon) and limestone (calcium carbonate) are added at the top of the blast furnace. Blasts of hot air are blown into the furnace near the bottom.
The figure below shows a series of chemical reactions that take place in the blast furnace.
Extracting Iron from Haematite (cont.)
The figure below shows what happens after carbon monoxide is formed.
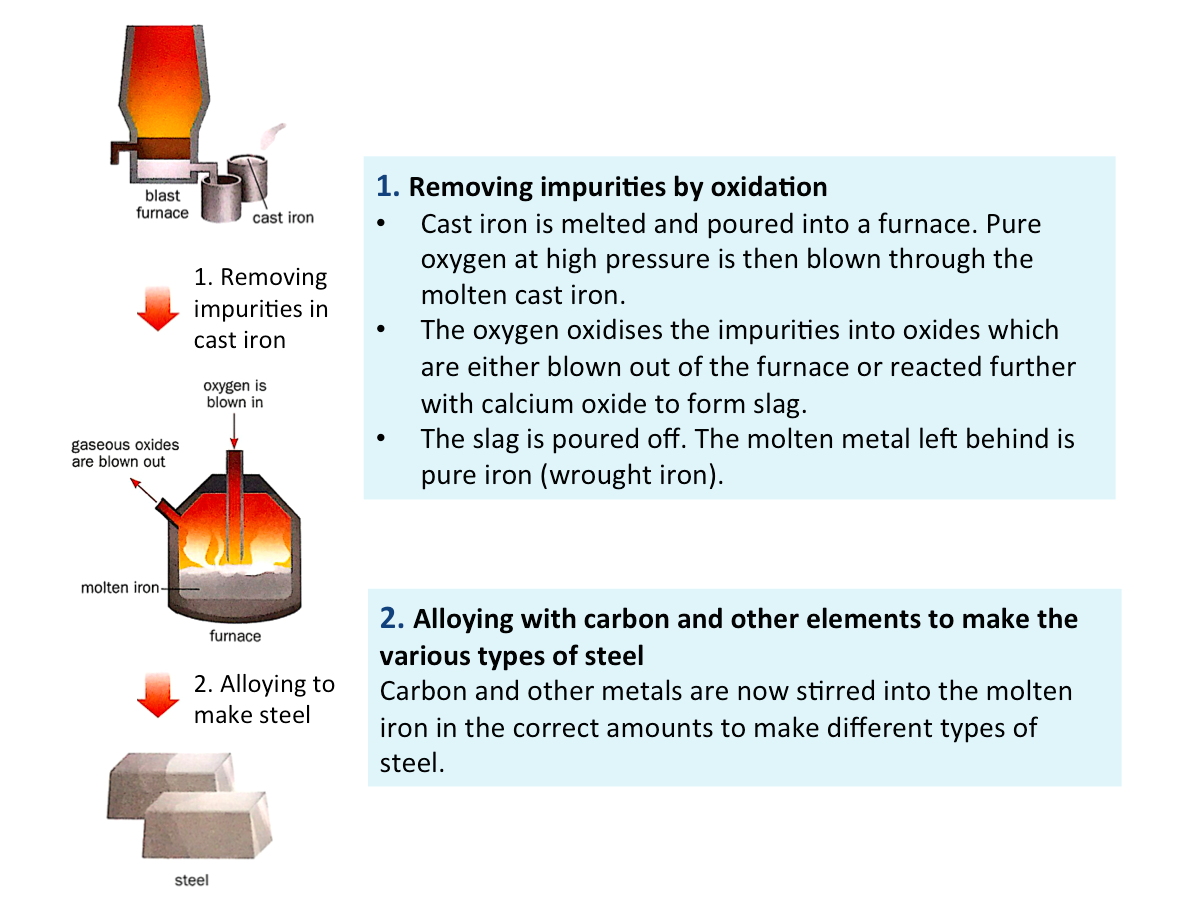
What is steel?
Steel is an alloy of iron with carbon and/or other metals. It is made from cast iron, involving two main stages: oxidation and alloying.
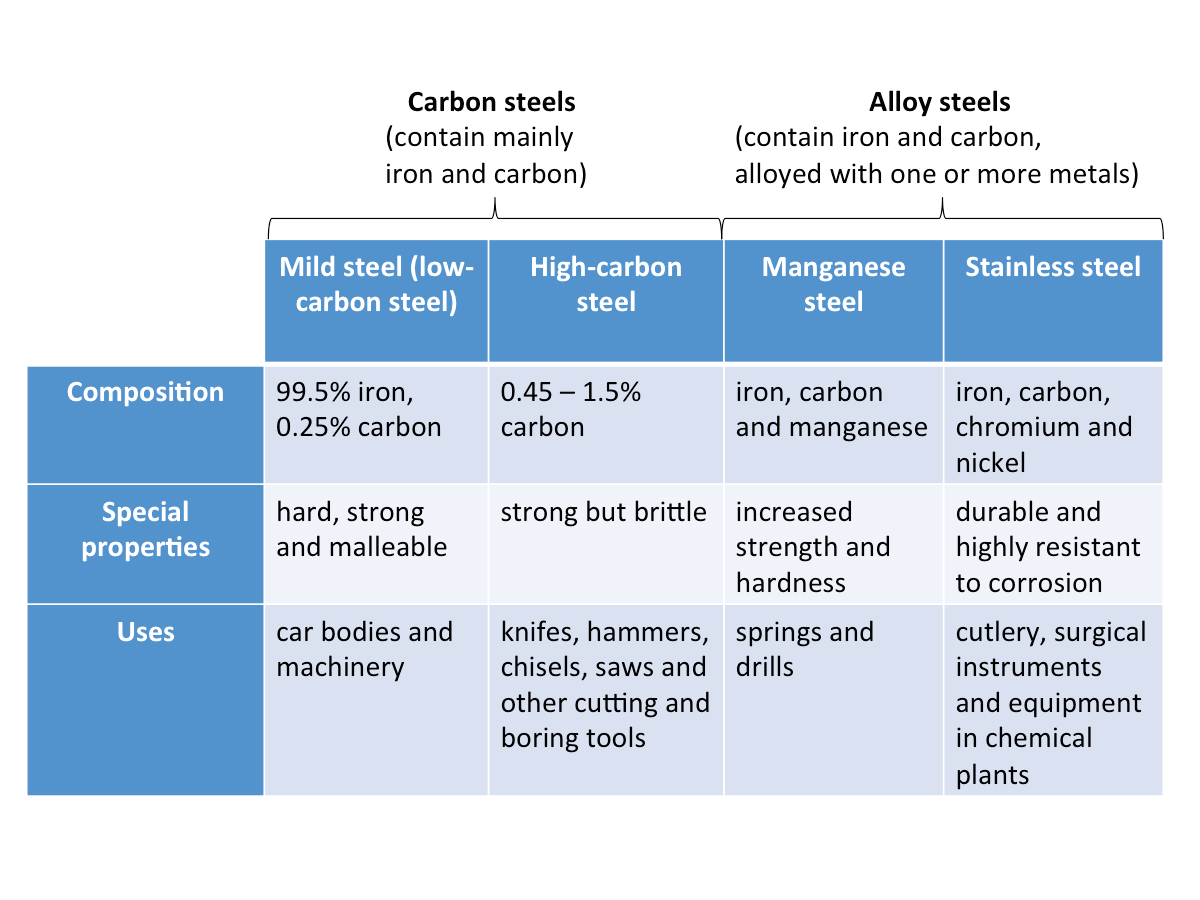
Different Types of Steel and Their Uses
The figure below shows some different types of steel and their uses.
What is rusting?
Rusting or the corrosion of iron is the slow oxidation of iron to form hydrated iron(III) oxide (rust). The equation for rusting is:
iron + oxygen + water --> hydrated iron(III) oxide
4Fe(s) + 3O2(g) + 2xH2O(l) --> 2Fe2O3.xH2O(s)
(The x in the chemical formula of hydrated iron(III) oxide indicates the number of water molecules present in the compound.)
Conditions for Rusting
The presence of both air (oxygen) and water are necessary for rusting to occur.
Sodium chloride and acidic substances such as sulfur dioxide and carbon dioxide speed up the rusting process.
Watch the video below to learn more about rusting and how to prevent it.
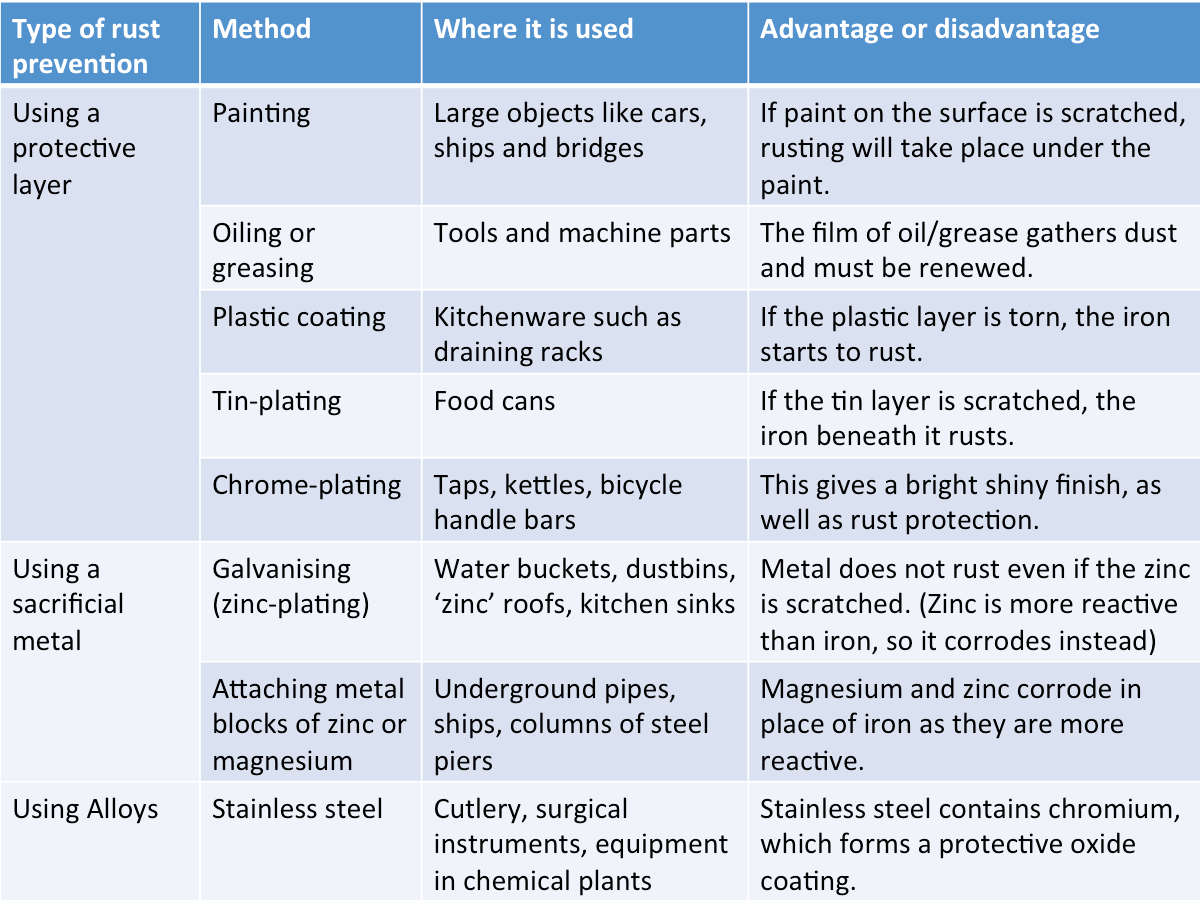
Rust Prevention
The table below summarises the common methods of rust prevention.
The Need for Recycling Metals
Metals are finite resources, meaning that they are limited in supply. With the increasing demand for metals, our natural resources will not last much longer. Hence, they need to be conserved.
Watch the video below to learn more about recycling metals.
How are metals recycled?
Lead, iron and aluminium are mainly recovered by scrap metal recycling.
Lead is recovered from car batteries. When a car battery no longer works, the lead inside it is reused. A large fraction of the iron and steel produced today is also recycled from scrap metal. Aluminium is recycled mainly from food and drink cans.
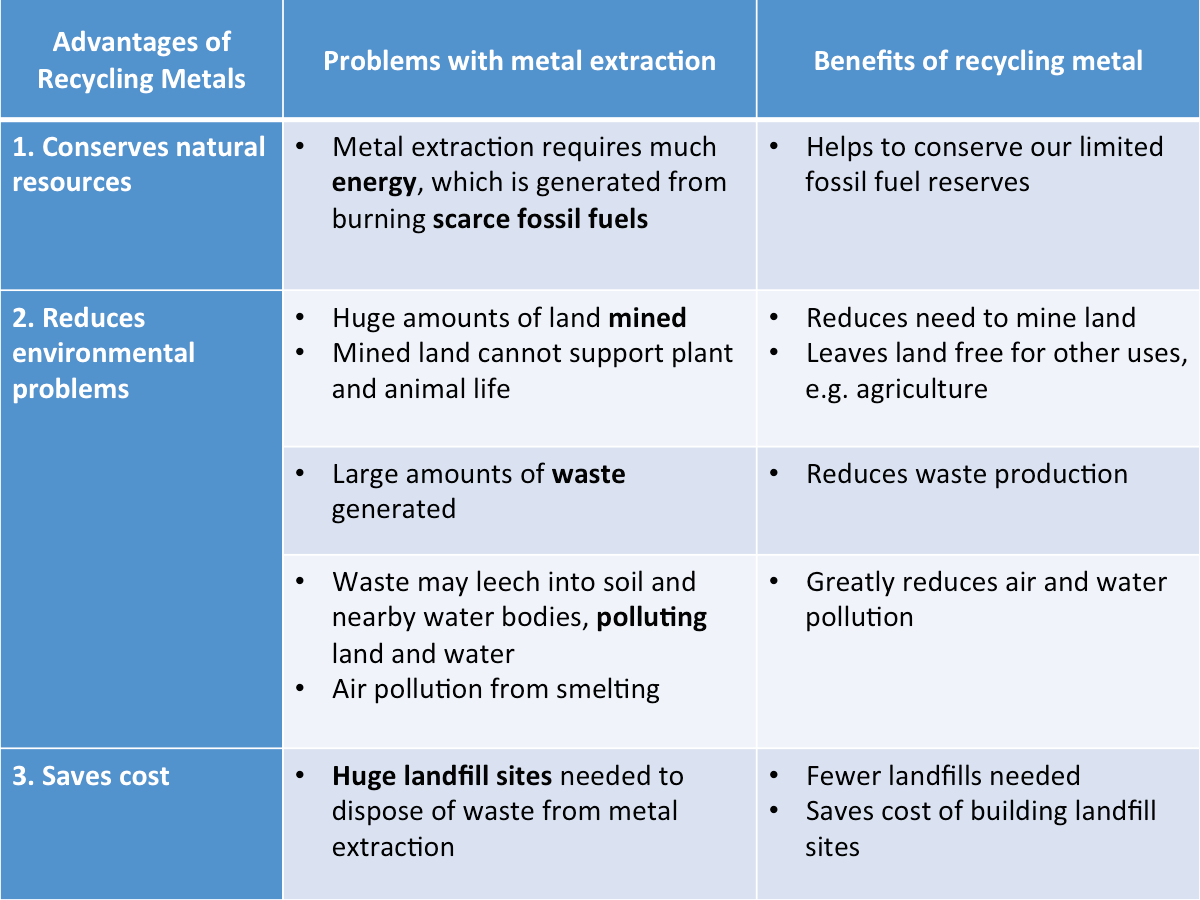
Advantages of Recycling Metals
The table below shows the advantages of recycling.
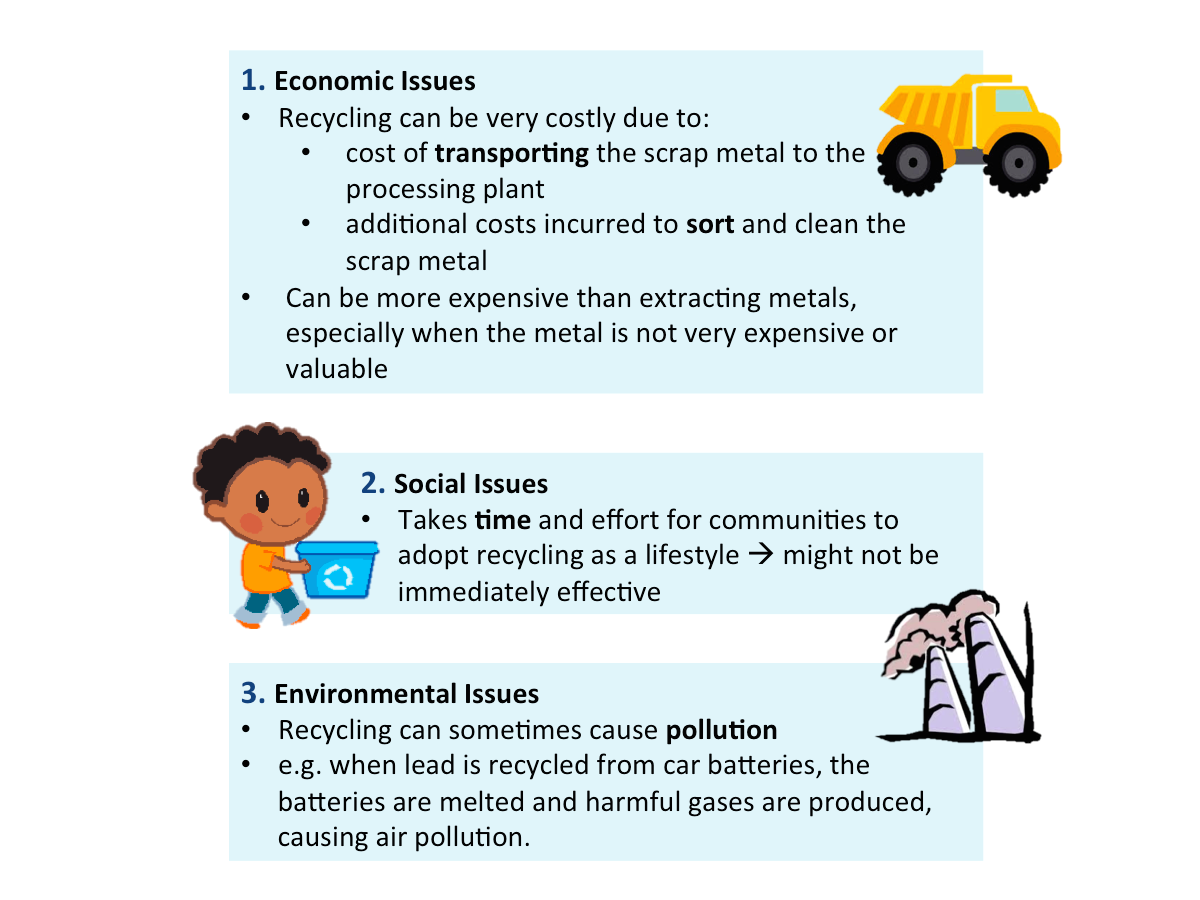
Issues Related to Recycling Metals
The figure below shows the issues related to recycling metals.