What are organic compounds?
Organic compounds, with a few exceptions, are compounds that contain the element carbon. Most organic compounds also contain hydrogen.
Organic compounds that contain only hydrogen and carbon are called hydrocarbons. Organic compounds may also contain other elements such as oxygen, chlorine and nitrogen.
Homologous Series
Organic compounds, with a few exceptions, are compounds that contain the element carbon. Most organic compounds also contain hydrogen. Organic compounds that contain only hydrogen and carbon are called hydrocarbons. Organic compounds may also contain other elements such as oxygen, chlorine and nitrogen.
A homologous series is a family of organic compounds that have:
- A general formula
- Similar chemical properties due to them having the same functional group
- Gradual change of physical properties (such as melting and boiling points, viscosity and flammability) as a result of increase in size and mass of the molecules.
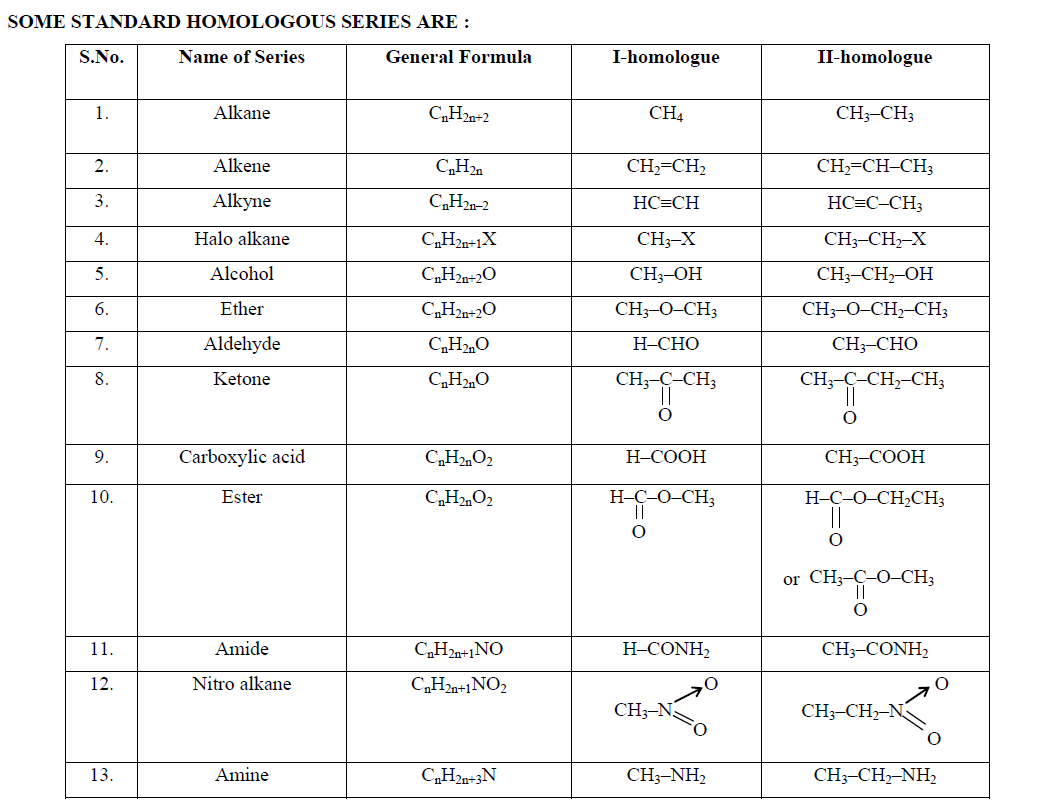
Examples of homologous series are alkanes,alkenes, alcohols and carboxylic acids.
Functional Group
A functional group is an atom or group of atoms that gives a molecule its characteristic properties.
Organic compounds in the same homologous series have similar chemical properties because they have the same functional group.
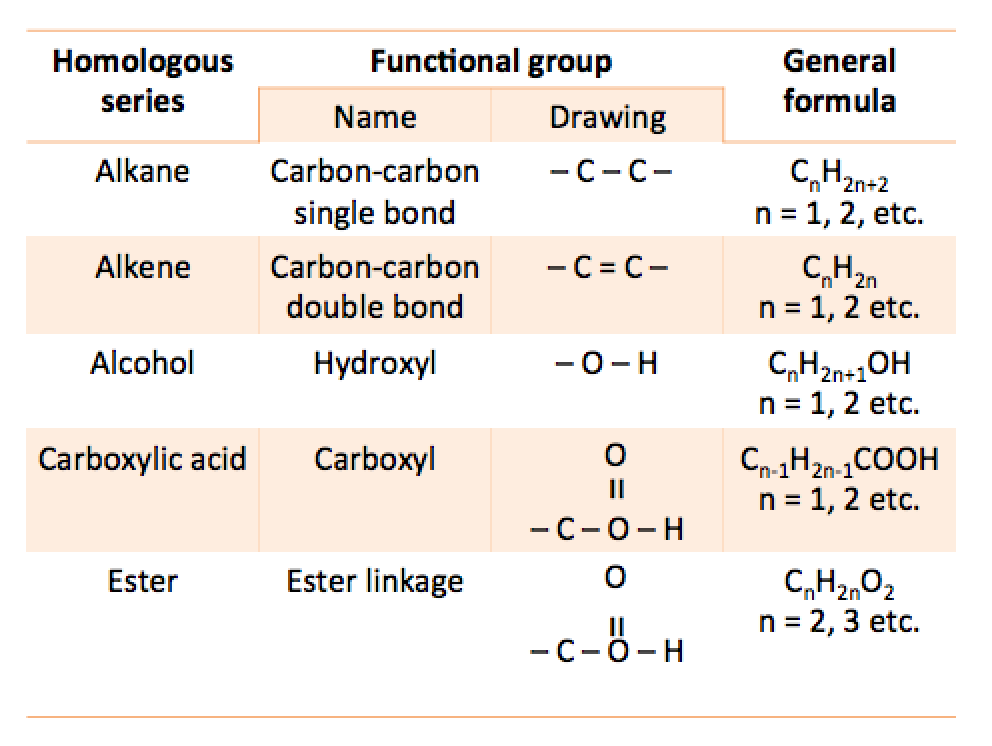
The table below shows four homologous series and their respective functional groups.
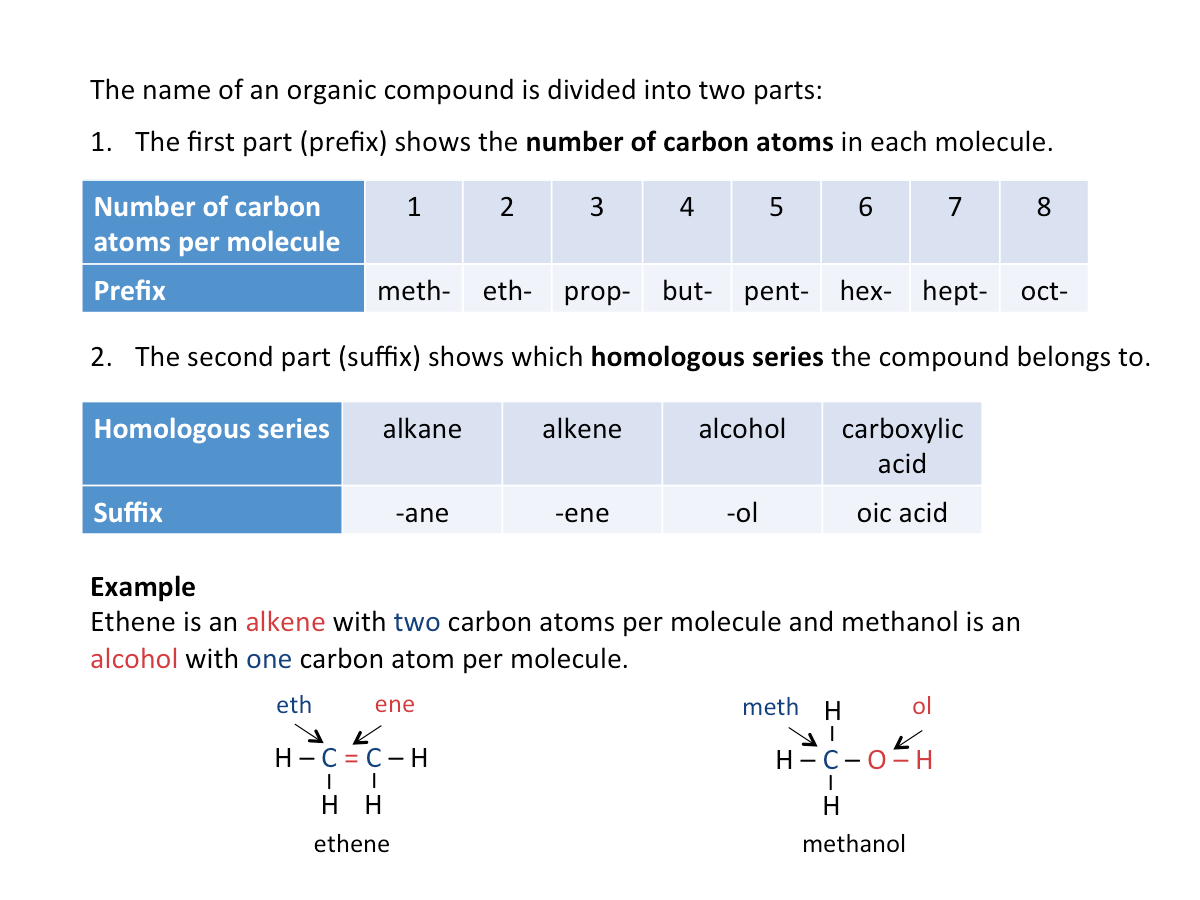
Naming Organic Compounds
Refer to the figure below for the naming of organic compounds.
What are they?
Petroleum (also called crude oil) and natural gas are fossil fuels. They are commonly used as sources of energy.
Petroleum is a naturally occurring mixture of hydrocarbons (mainly alkanes).
Natural gas is mostly made up of methane (about 70-90%), and other short-chain alkanes.
Watch the video below to learn more about crude oil and how it is separated.
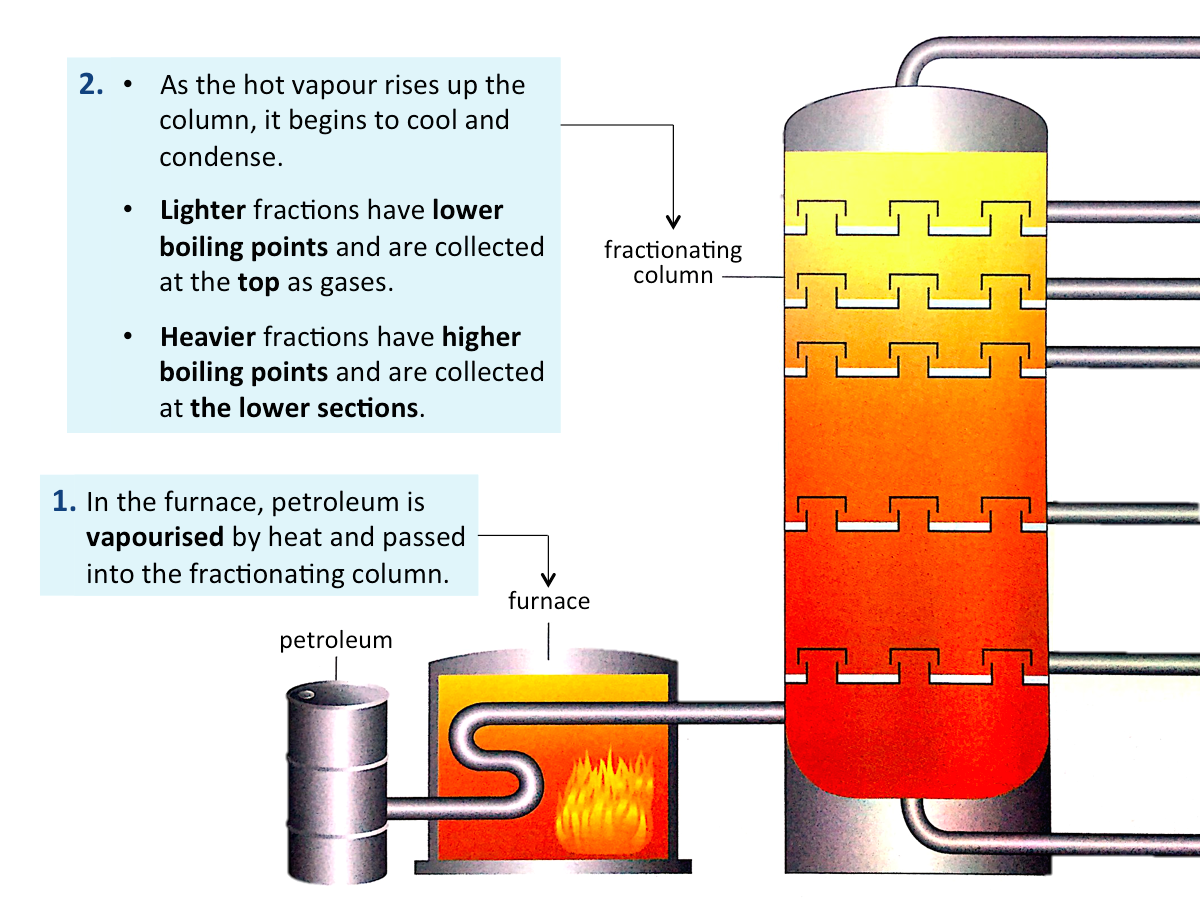
Fractional Distillation of Petroleum
Petroleum can be separated into useful fractions by fractional distillation.
Each petroleum fraction is a mixture of hydrocarbons which boils over a certain temperature range.
- Lighter fractions consist of smaller hydrocarbons.
- Heavier fractions consist of bigger hydrocarbons.
The figure below shows how the hydrocarbons in petroleum are separated in oil refineries.
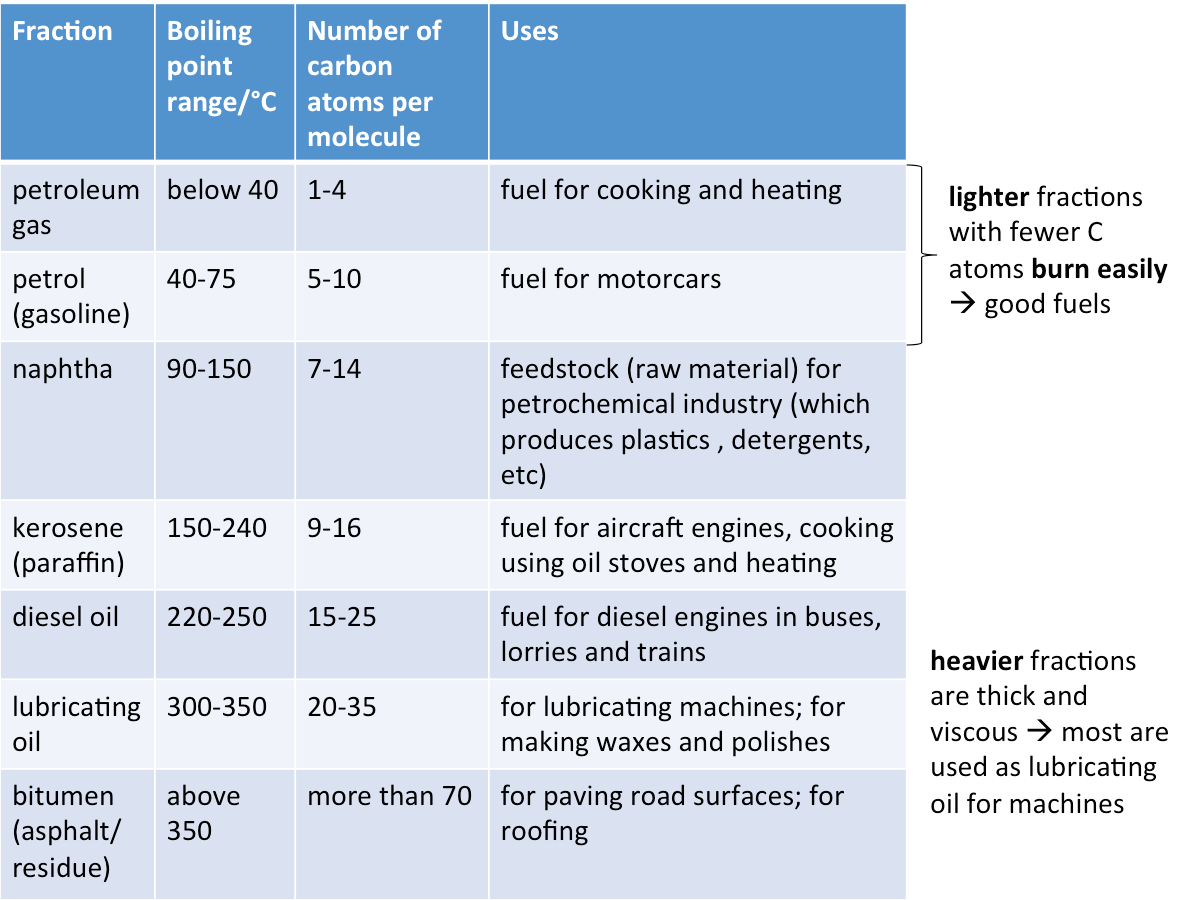
Uses of Petroleum Fractions
Refer to the table below for the uses of various petroleum fractions.
Uses of Petroleum Fractions (Video)
Alternatively, you can watch the video below to learn about the uses of petroleum fractions.
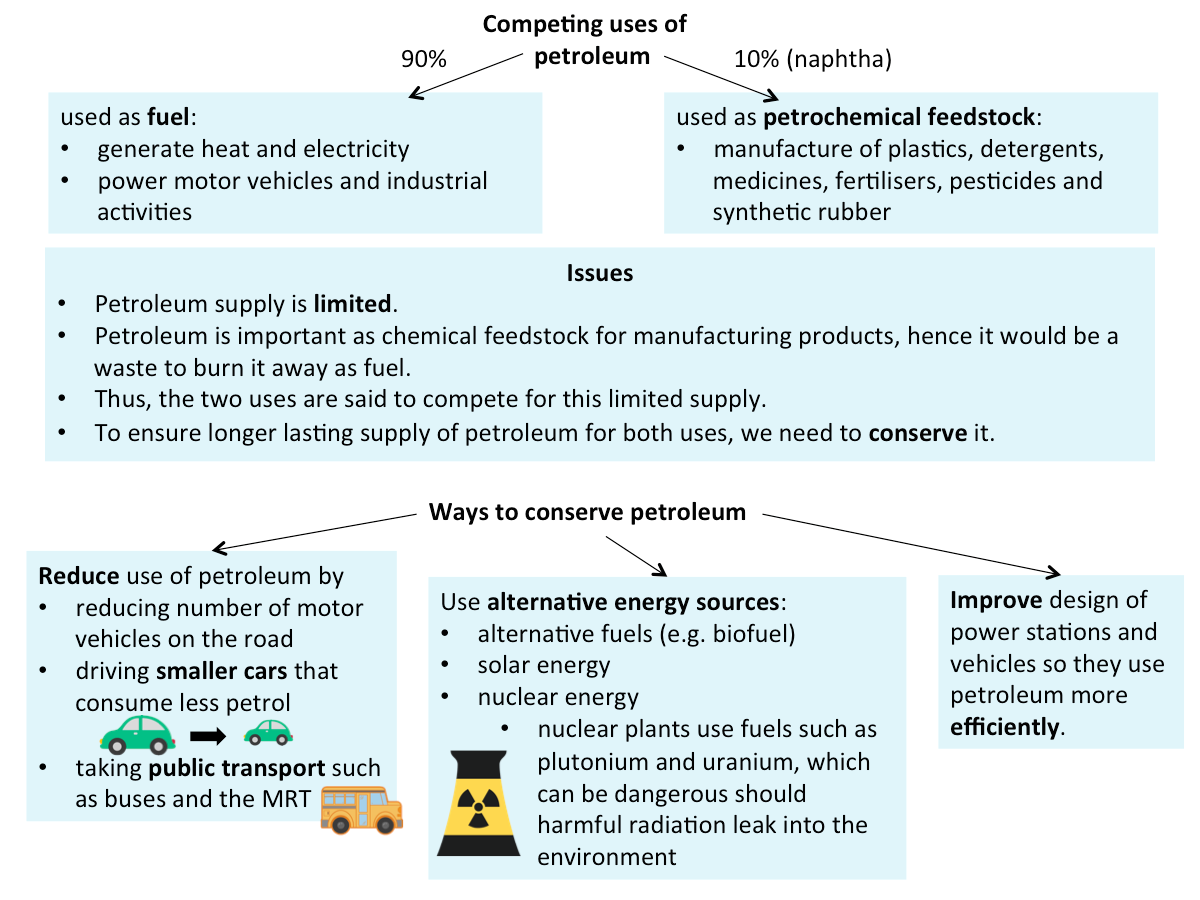
Issues Related to the Uses of Petroleum
The figure below shows the issues related to the uses of petroleum.
What are alkanes?
Alkanes are a homologous series of saturated hydrocarbons:
- They have the general molecular formula CnH2n+2 (where n is the number of carbon atoms and = 1, 2, 3, etc.)
- Contains carbon-carbon single covalent bonds.
- Have similar chemical properties
Saturated means that the molecule contains only carbon-carbon single bonds. Each carbon atom is covalently bonded to four other atoms, so all the outer electrons of each carbon atom are used in formation of single bonds, and no new atom can be added to it anymore.
They are hydrocarbons, meaning they contain only elements carbon and hydrogen.
Watch the video below to learn more about alkanes (0:00-3:10).
Naming Alkanes
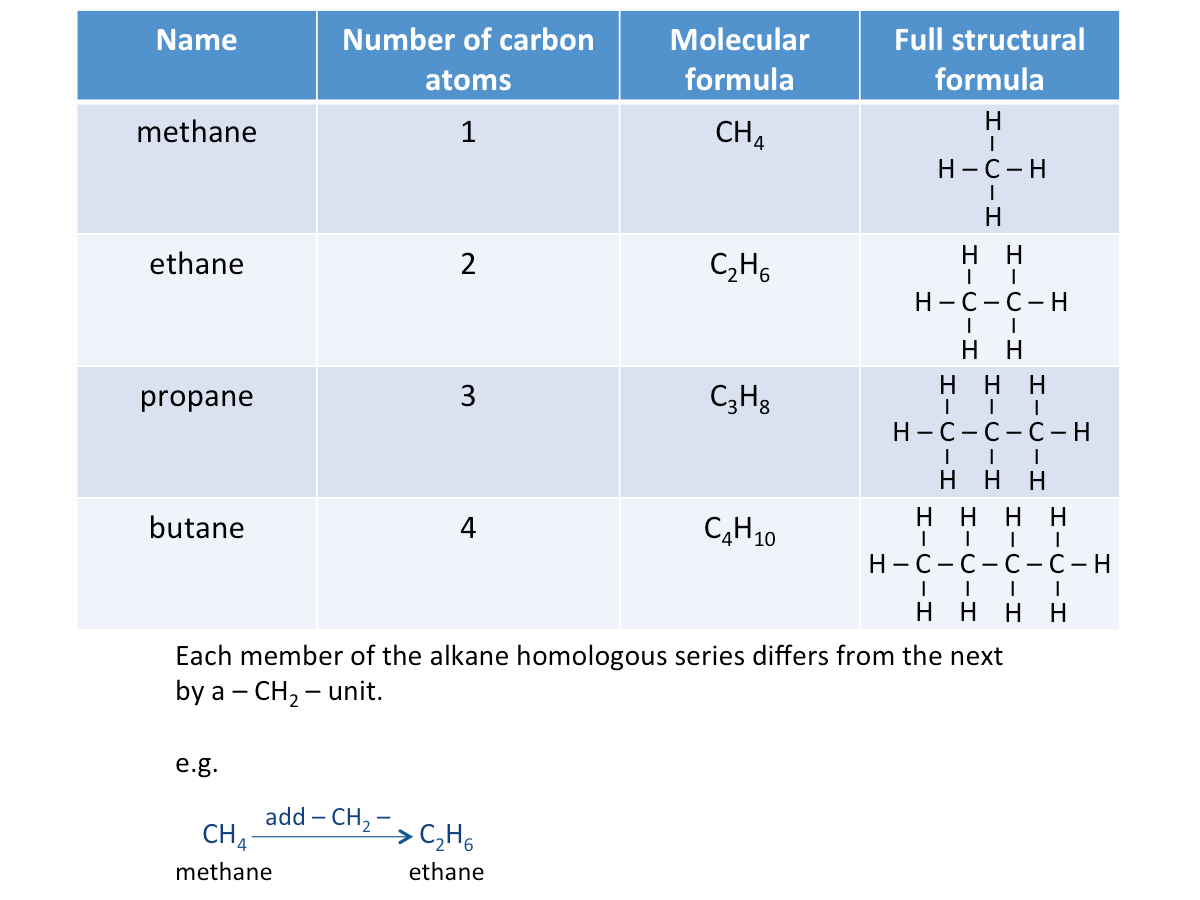
The name of an alkane ends with -ane. The table below shows the first four members of the alkane homologous series.
Unsaturated Hydrocarbons
Any molecule that contains carbon-carbon double bonds is unsaturated. Thus,
alkenes are unsaturated hydrocarbons.
Structural Formulae of Alkanes
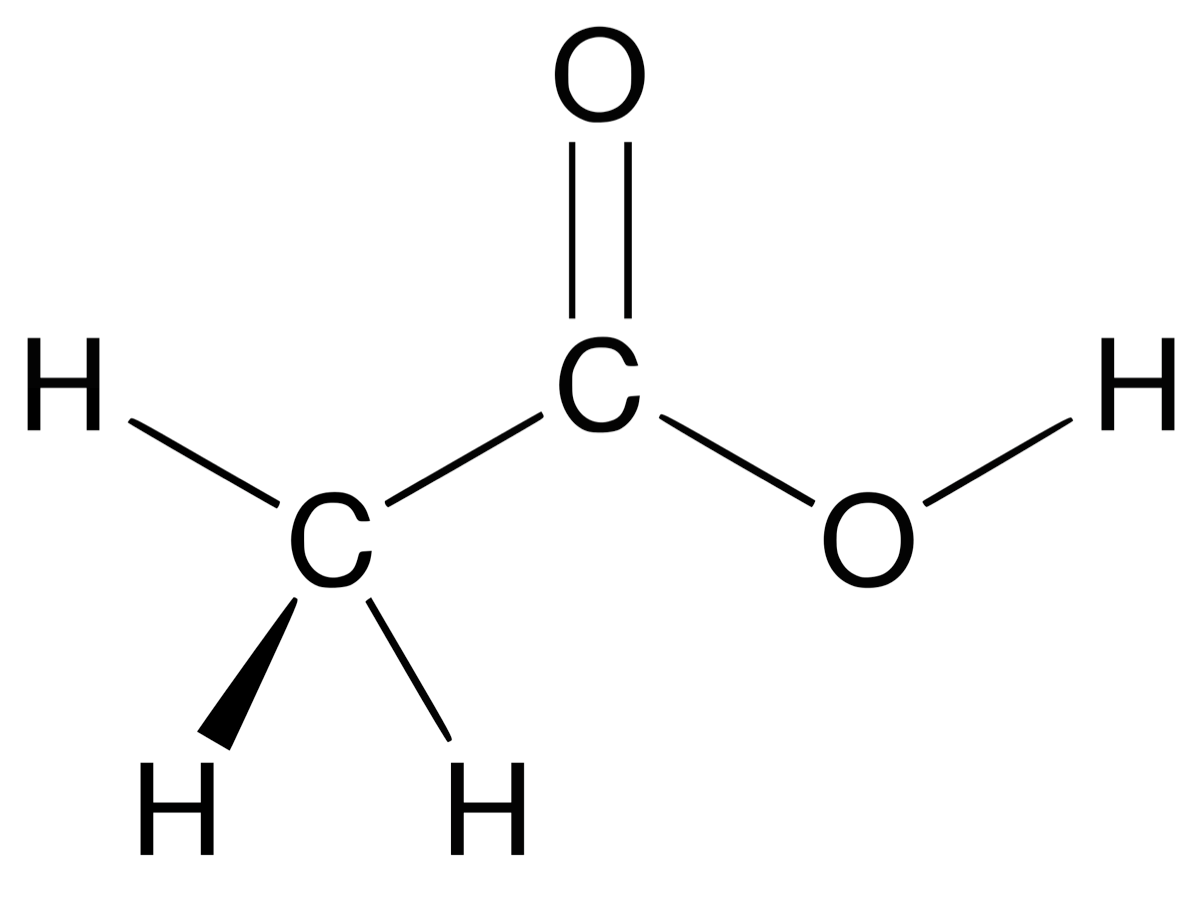
A structural formula is the formula which shows how atoms are arranged in a molecule. The full structural formula or displayed formula shows all the bonds between the atoms in a molecule. Watch the video below to learn the different ways of naming organic compounds.
Physical Properties of Alkanes
Alkanes are covalent comopunds with simple molecular structures. Thus, they have the properties of typical covalent compounds.
Alkanes
- Have low melting and boiling points
- Are insoluble in water
- Are soluble in organic solvents
How do the physical properties of alkanes change going down the series?
1. They become more viscous.
As the molecular sizes of alkanes increase, the forces of attraction between the molecules increase, causing the alkanes to become more viscous i.e. flow less easily.
2. They become less flammable.
Alkanes are flamamble and burn easily in air. As the sizes of the alkane molecules increase, the percentage of carbon in the alkane molecules increases. Hence, alkanes become less flammable. A smokier flame caused by the incomplete combustion of alkane is also produced.
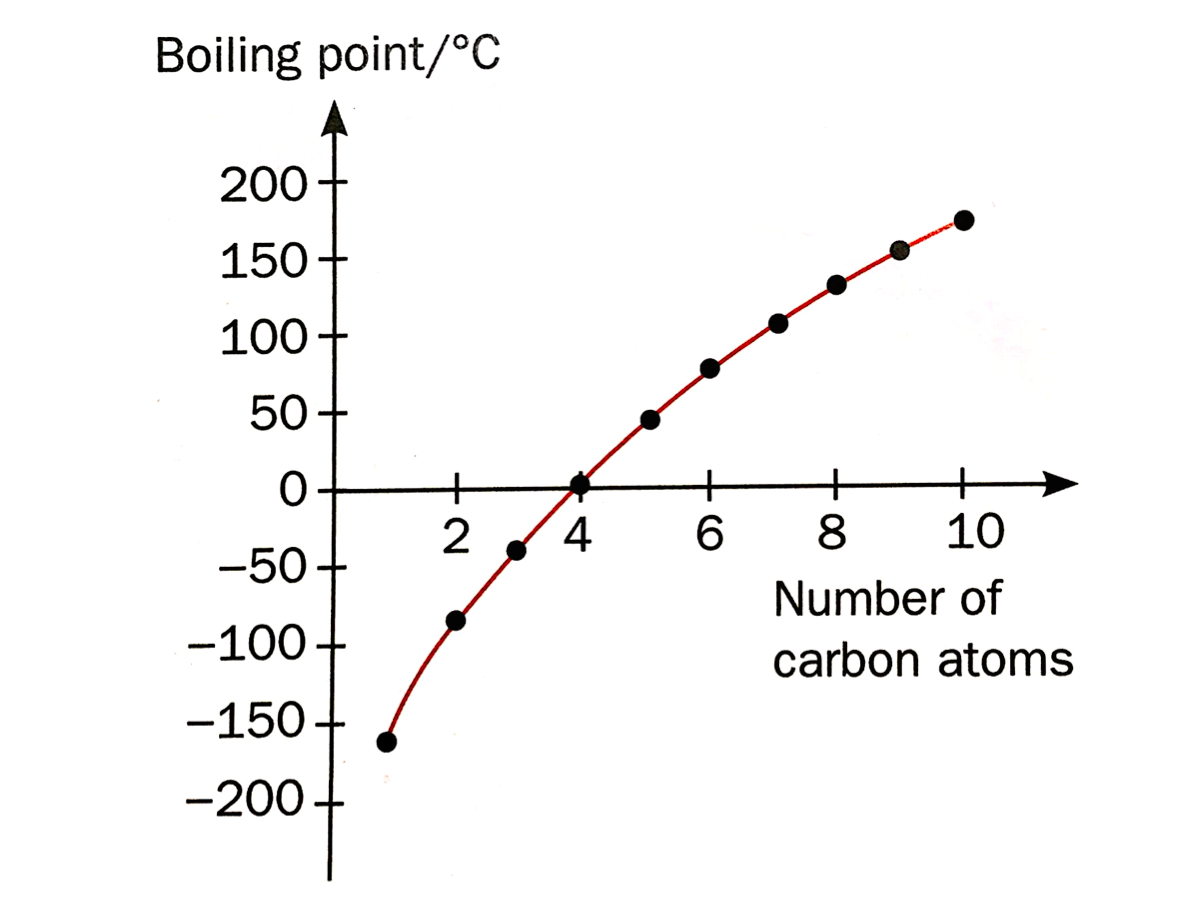
3. Their melting and boiling points increase.
As the molecular sizes of alkanes increase, the forces of attraction between the molecules increase, causing the melting and boiling points to increase.
The graph below shows the trend in boiling points of alkanes as the number of carbon atoms increases.
Chemical Properties of Alkanes - Combustion
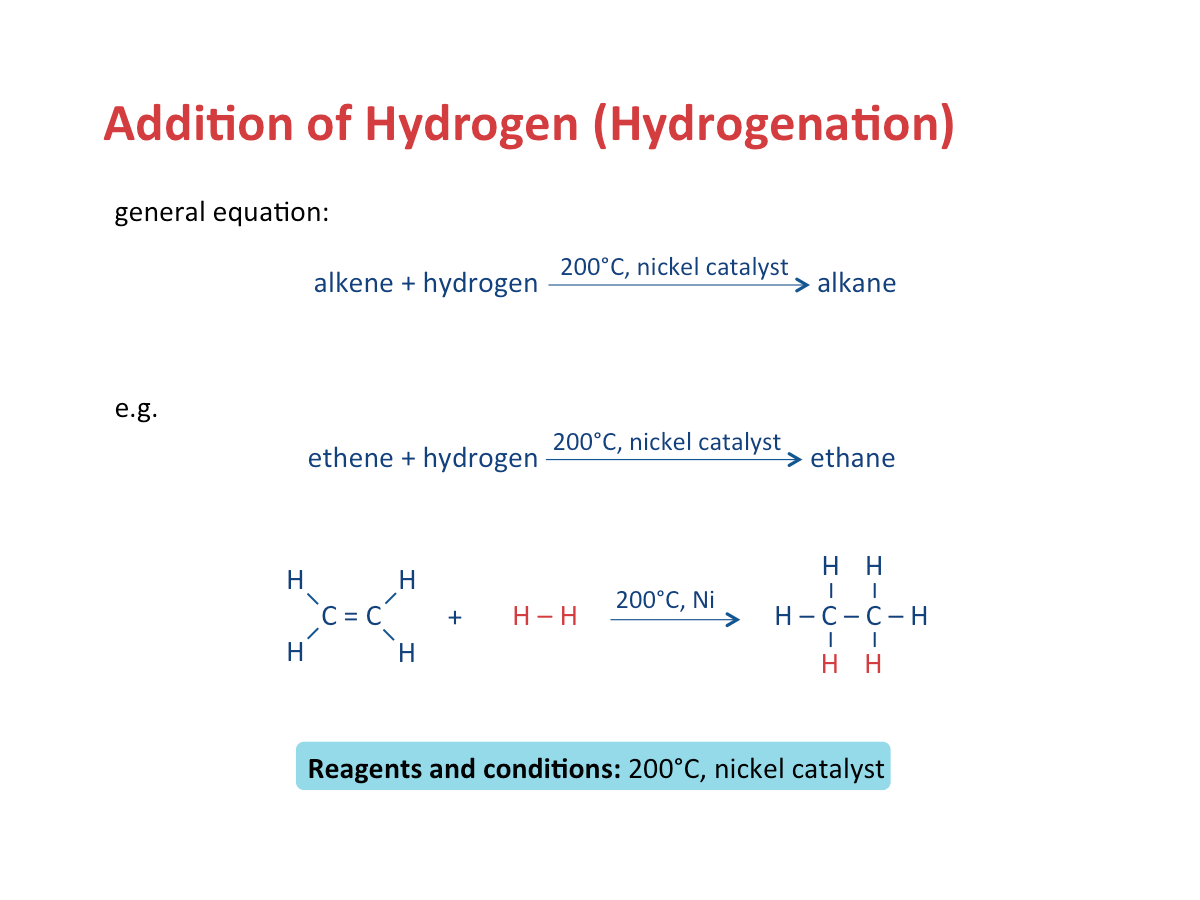
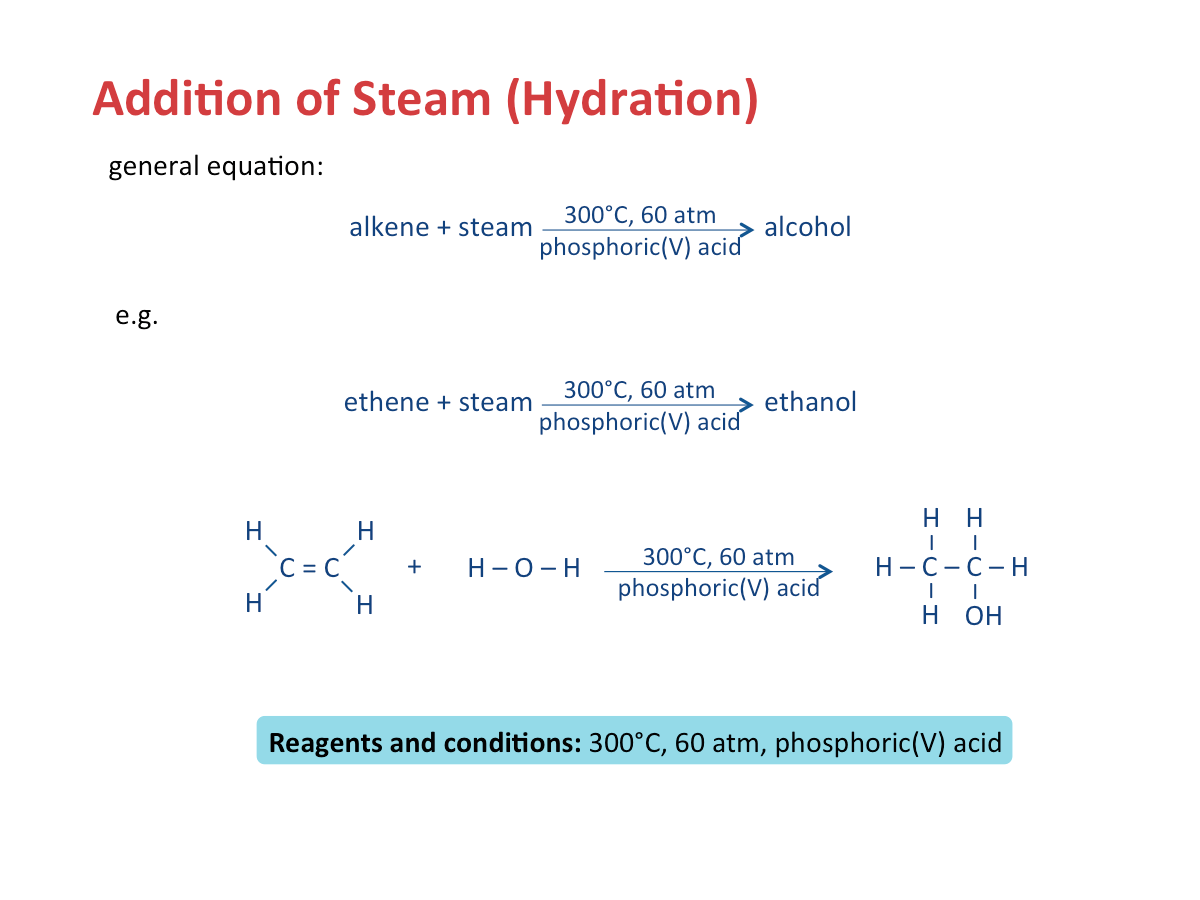
Alkanes are generally unreactive as they consist of only carbon-carbon single (C-C) bonds and carbon hydrogen (C-H) bonds which are strong and difficult to break. However, alkanes can undergo (1) combustion, (2) substitution, and (3) cracking reactions. alkane + oxygen? --> carbon dioxide + water Alkanes react with halogens, such as chlorine and bromine, in the presence of ultraviolet (UV) light, as the catalyst. Each hydrogen in an alkane is displaced one at a time by one molecule of halogen gas. Cracking is the breaking down of large alkane molecules to produce smaller useful molecules. It is carried out by using high pressures and temperatures without a catalyst. It can also be down in lower temperatures and pressures in presense of a catalyst. It is a very important industrial process because it can cater to the demand for fuels by producing smaller useful molecules. Alkenes are a homologous series of unsaturated hydrocarbons: The name of an alkene ends with -ene. The table below shows the various formulae of the first three alkenes in the homologous series. When the size and mass of alkene molecules increases, a gradual change in the following properties is observed as well: Combustion In this process, alkenes react with hydrogen to form alkanes at high temperature (e.g. 200 Bromine gas or bromine water is brown in colour. Addition of bromine is an important test to distinguish alkanes from alkenes. Steam and alkenes are passed over a catalyst such as concentrated phosphoric acid at high temperature (about 300 Polymerisation is a process of joining small identical units to form large molecules. Alkenes are unsaturated. They can react with each other to produce a long molecule through polymerisation. Cracking is the breaking down of long-chain hydrocarbons into smaller molecules. 1. Cracking is used to produce short-chain alkenes. The table below shows the similarities and differences between an alkane and an alkene. Polyunsaturated fats or oils contain more than one carbon-carbon double bond. Saturated fats, on the other hand, consist of only carbon-carbon single bonds.
Combustion- Complete
When ignited by a spark or flame, alkanes burn with blue flame without smoke, readily in excess air (oxygen) to produce carbon dioxide and water vapour.
alkane + oxygen --> carbon dioxide + water vapour
The combustion of alkanes is highly exothermic.
Combustion - Incomplete
During incomplete combustion, alkanes burn with a yellow smoky flame.
Possible products: Soot (carbon), carbon monoxide, and water
E.g. Incomplete combustion of methane in air may give:
(1) Methane + oxygen --> carbon monoxide + water
2 CH4(g) + 3 O2(g) --> 2 CO(g) + 4 H2O(l)
(2) Methane + oxygen --> soot (carbon) + water
CH4Chemical Properties of Alkanes - Substitution
Overall chemical equation: CH4 + 2 Cl2 --> CH2Cl2 + 2 HCl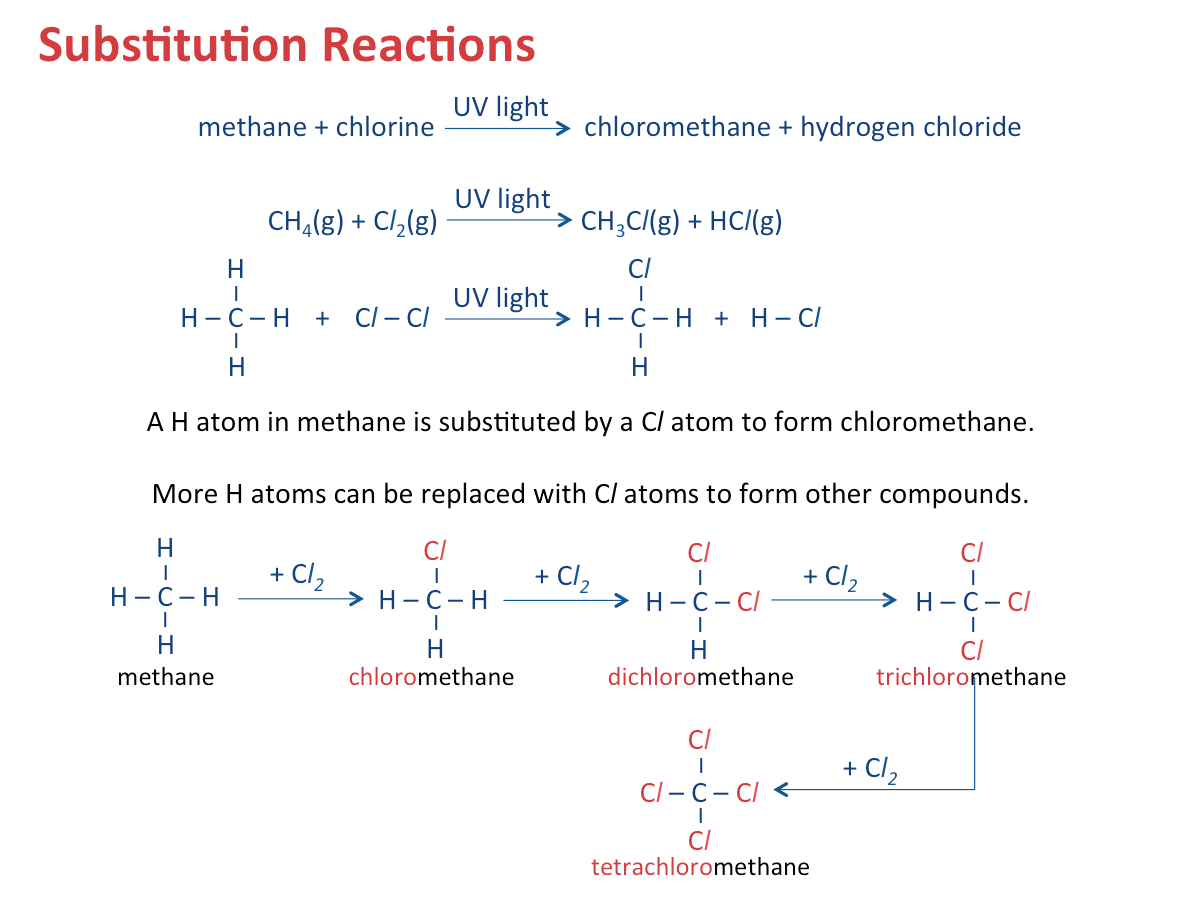
Chemical Properties of Alkanes - Cracking
Possible reaction 1: Large alkane --> smaller alkane + alkenes
Possible reaction 2: Large alkane --> alkenes + hydrogen
Try the following questions and click on the coloured shapes to reveal the answers!What are alkenes?
- They have a general formula CnH2n, where n is the number of carbon atoms.
- Contains one or more carbon-carbon double bonds as their functional group.
- Similar chemical properties.
- A gradual change in their physical properties.
Unsaturated means that the molecule containts carbon-carbon double bonds (C=C).
- In carbon-carbon double bond, each carbon atom in this type of bond is covalently bonded to three atoms only, so a new atom can be added to it.
- Each member differs by 1 C atom and 2 H atoms.
Watch the video below to learn more about alkenes (3:10-5:53).Naming Alkenes
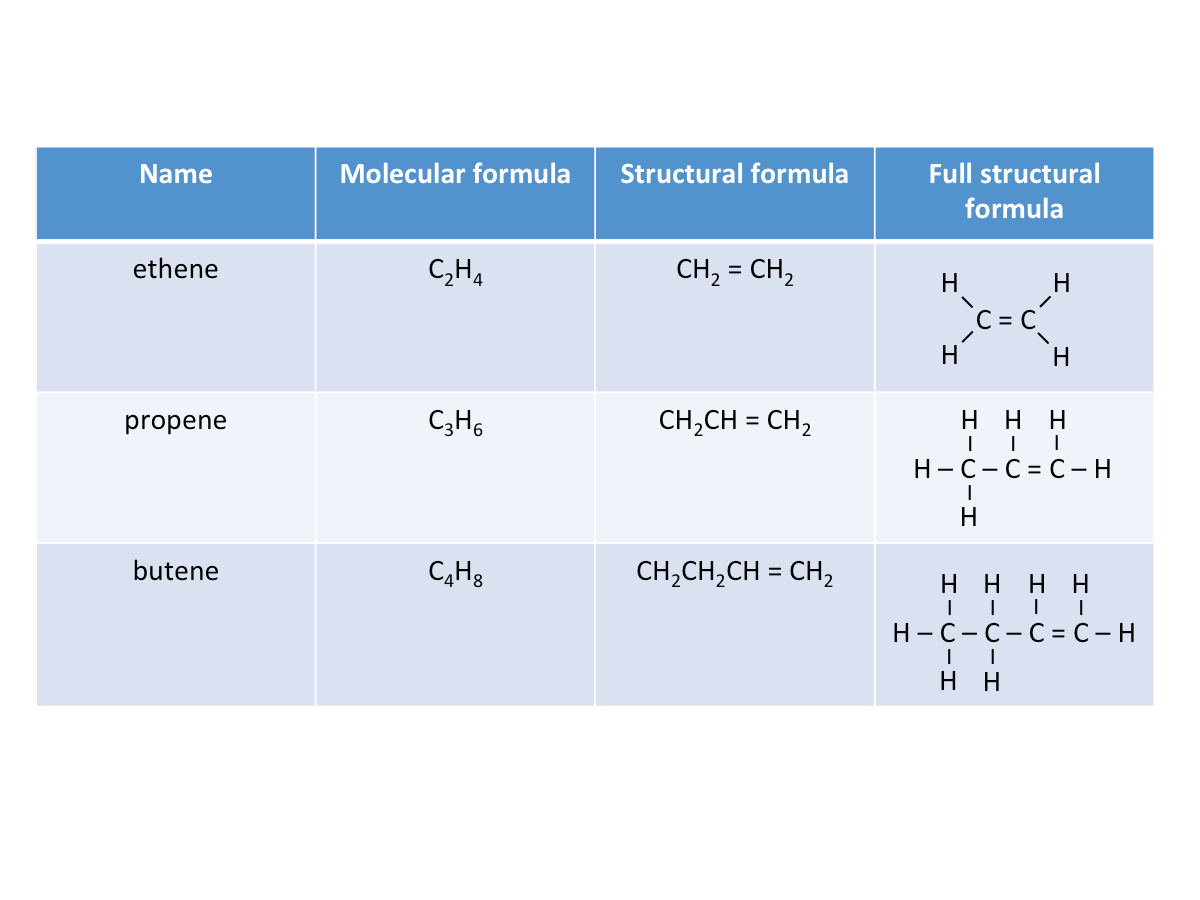
Physical Properties of Alkenes
- Boiling point increases
- Density increases
- Viscosity increases
- Flammability decreases
Are you able to come up with the explanation for each physical property? Click on Reveal Explanation to check your answers!Combustion and Addition Reactions
This reaction is exothermic. In complete combustion, alkenes burn in excess air (oxygen) to form carbon dioxide and water vapour:
alkene + oxygen --> carbon dioxide + water vapour
Alkenes burn with sootier flames than alkanes because the percentage of carbon by mass in alkenes is higher than that in alkanes for the same number of carbon atoms present in their respective molecules.
Addition Reactions
Alkenes are generally reactive because they are unsaturated (due to the carbon-carbon double bonds). New atoms can be added to the carbon atoms that form the double bond, hence they readily undergo addition reactions - 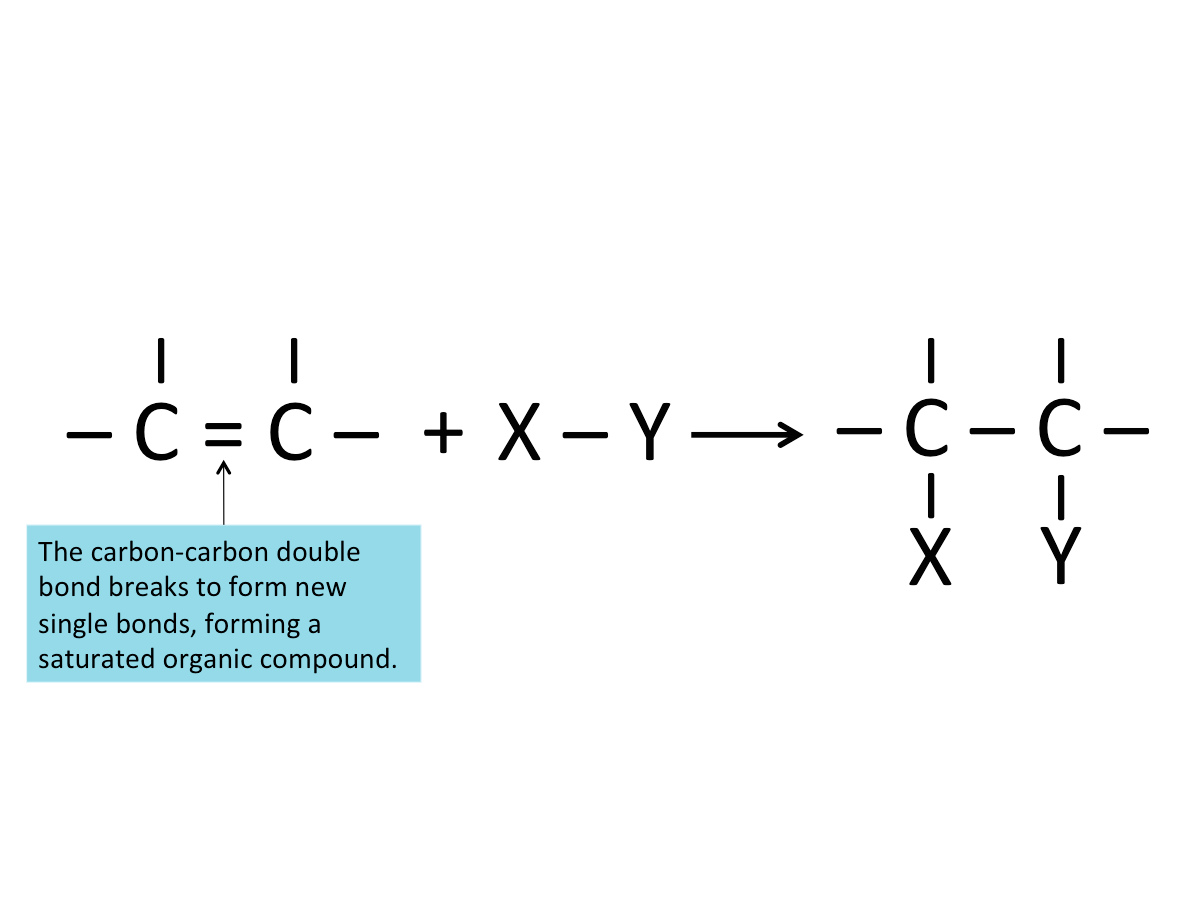
Addition of Hydrogen (Hydrogenation)
Addition of Bromine (Bromination)
When an alkene is added to a solution of reddish-brown bromine, bromine is decolourised and a colourless liquid is formed (in alkanes, bromine remains brown as no reaction will take place). 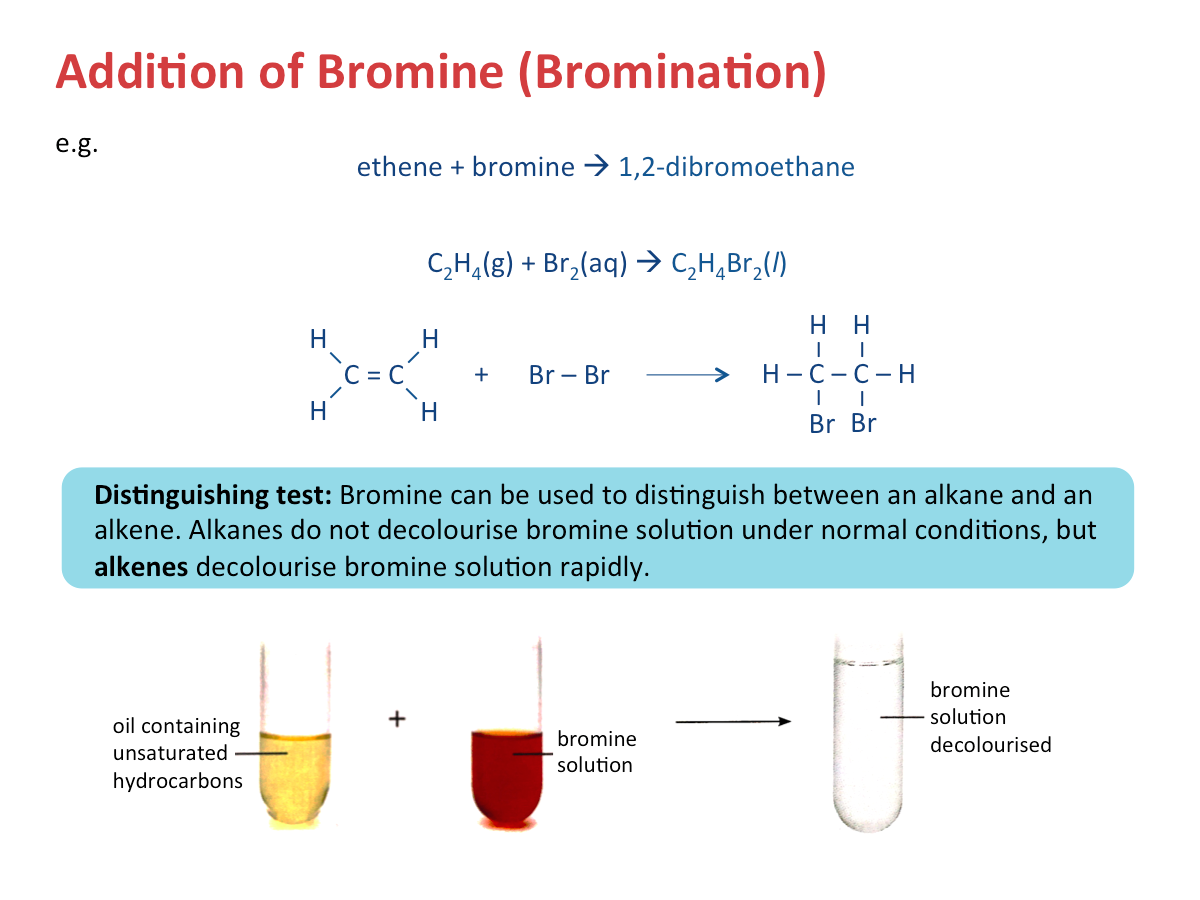
Addition of Steam (Hydration)
Addition Polymerisation
For example, ethene molecules will react with one another to produce polyethene. 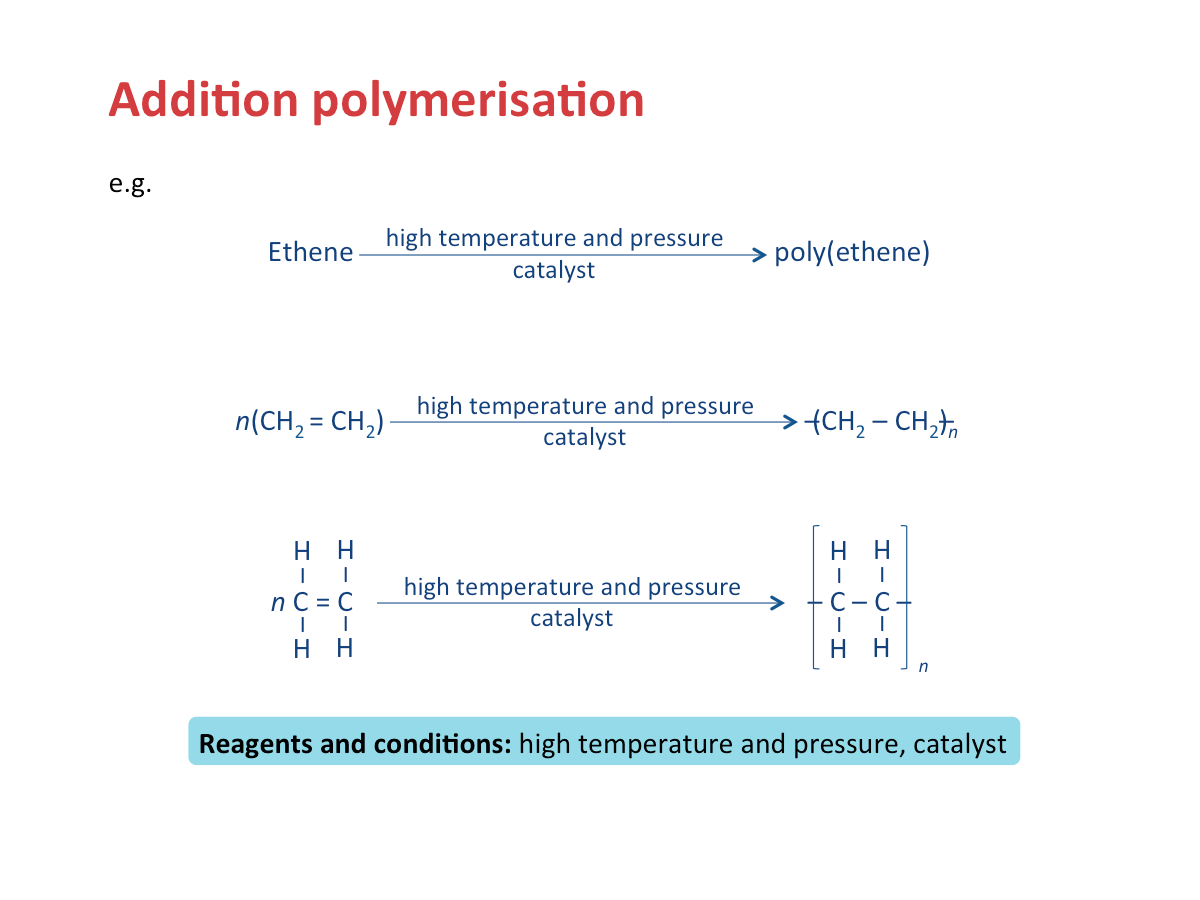
What is cracking?
Alkenes are obtained by cracking petroleum.
A catalyst may be used to speed up the process of cracking. This process is known as catalytic cracking.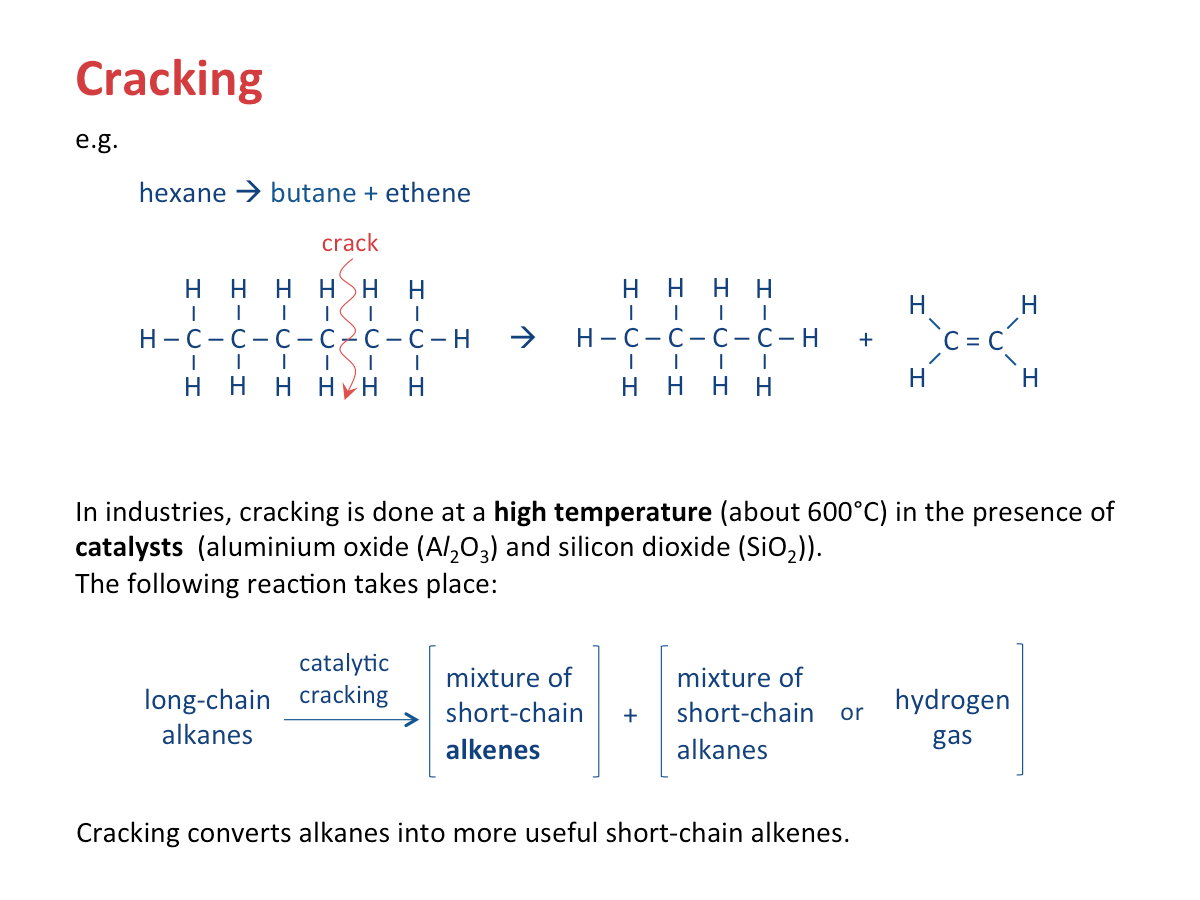
Importance of Cracking
long-chain alkane --> shorter-chain alkane + short-chain alkene
Short-chain alkenes are used as starting materials for making ethanol and plastics.
2. Cracking is used to produce hydrogen.
e.g.C18 alkane molecule --> C8 alkene molecule + C10 alkene molecule + hydrogen
C18H38 --> C8H16 + C10H20 H2
Hydrogen is a by-product in the cracking of alkanes.
3. Cracking is used to produce petrol.
Hydrocarbons of high molecular mass can be converted by catalytic cracking into smaller molecules which are in higher demand (e.g. petrol).
The video below summarises the key ideas of hydrocarbon cracking. What are the similarities and differences between saturated and unsaturated hydrocarbons?
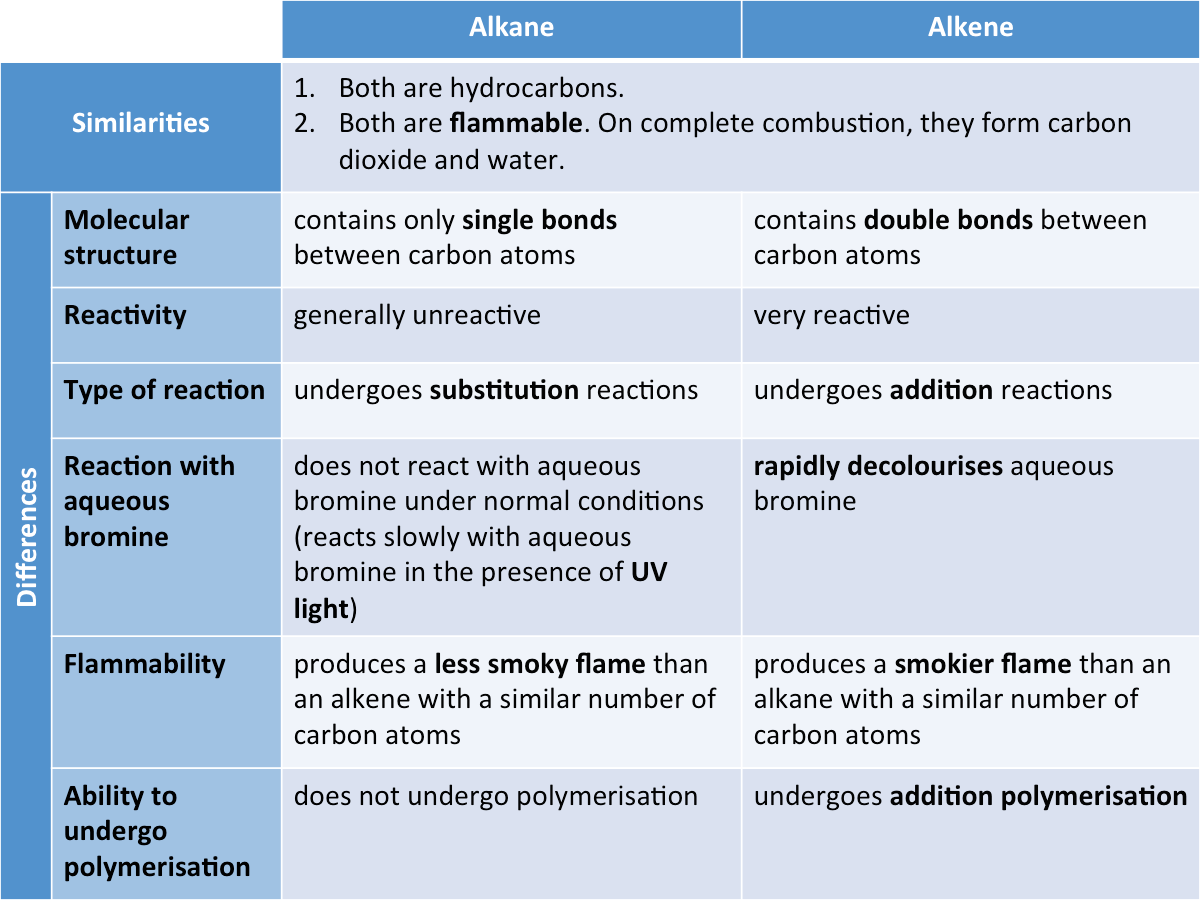
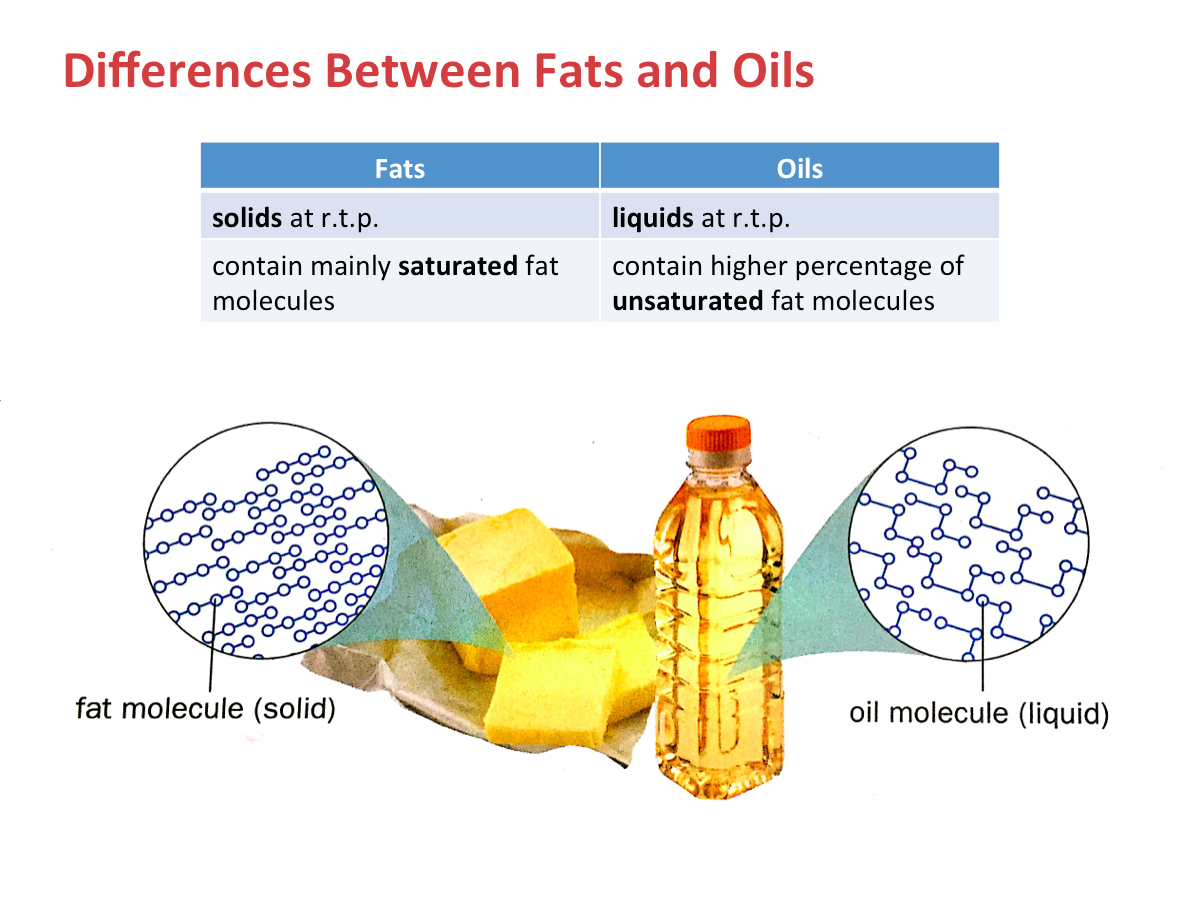
What does polyunsaturated mean?
Hydrogenation is used to convert unsaturated fats into saturated. For example, vegetable oils are polyunsaturated, so they exist as liquid at room temperature generally. In making margarine, they are hardened to form margarine by adding hydrogen to some of the carbon-carbon double bonds (C=C).