Enzymes
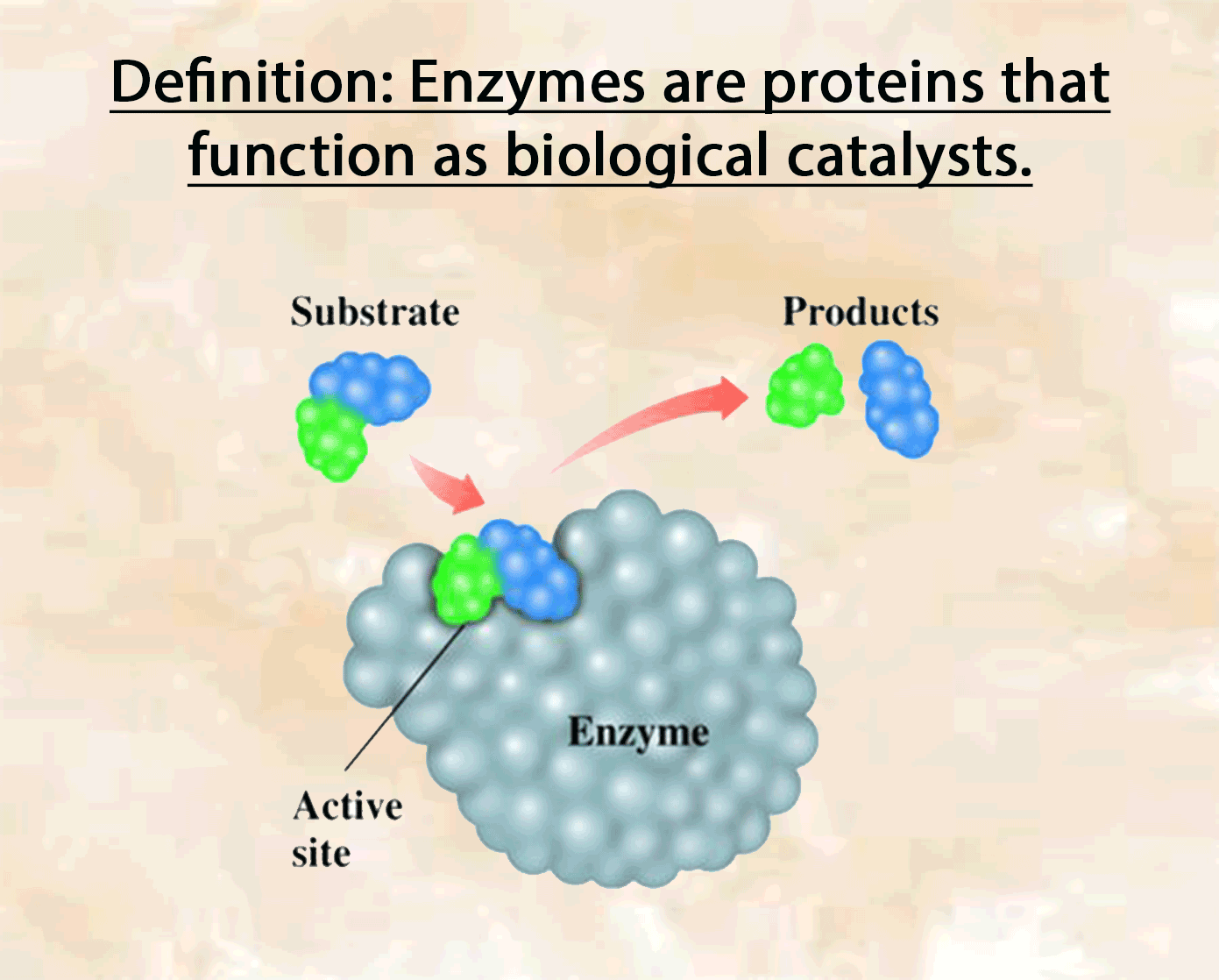
Definition of enzyme:
Catalyst
Definition of catalyst:
A catalyst is a substance that increases the rate of a chemical reaction and is not changed by the reaction.
[Supplement] Enzyme Action
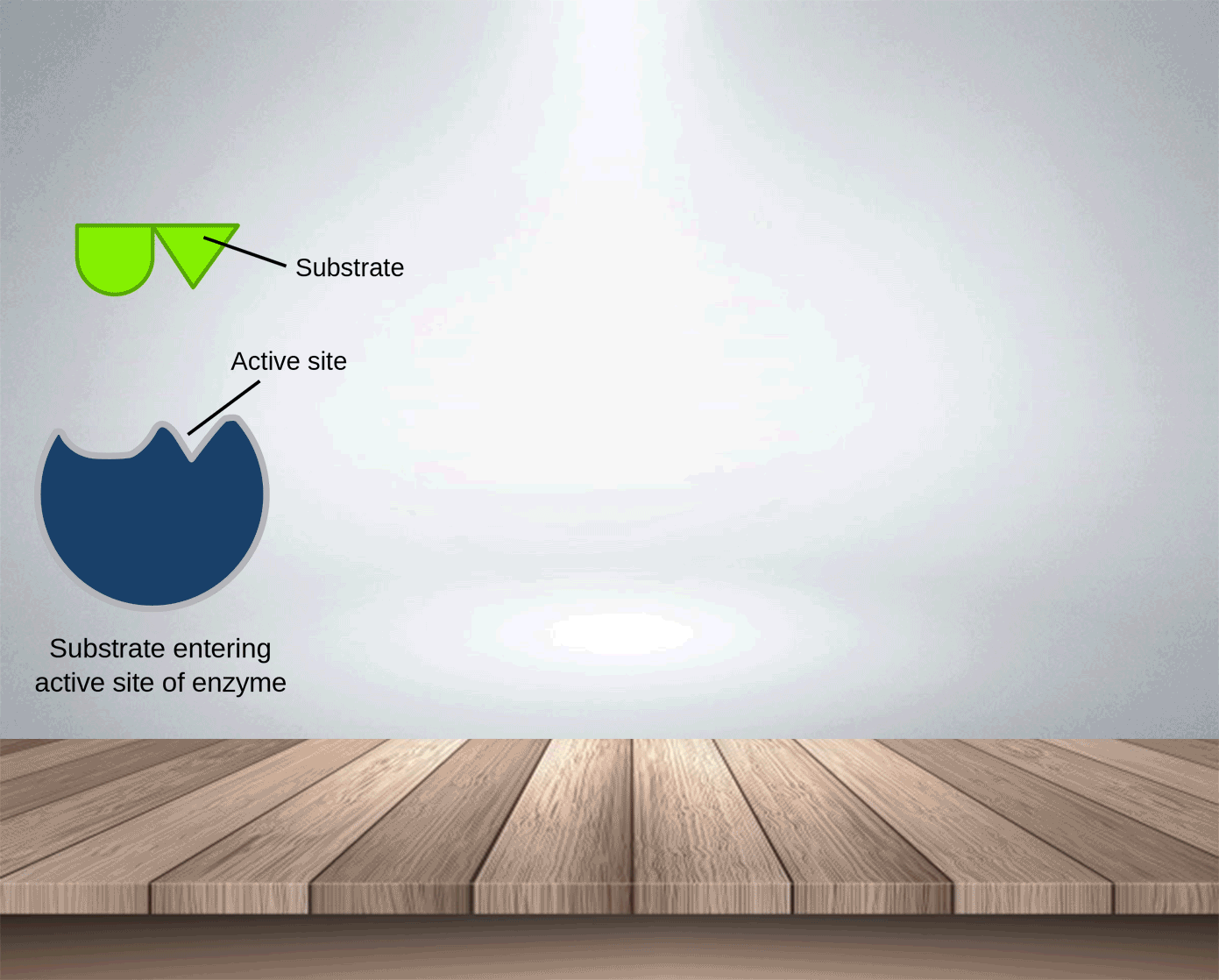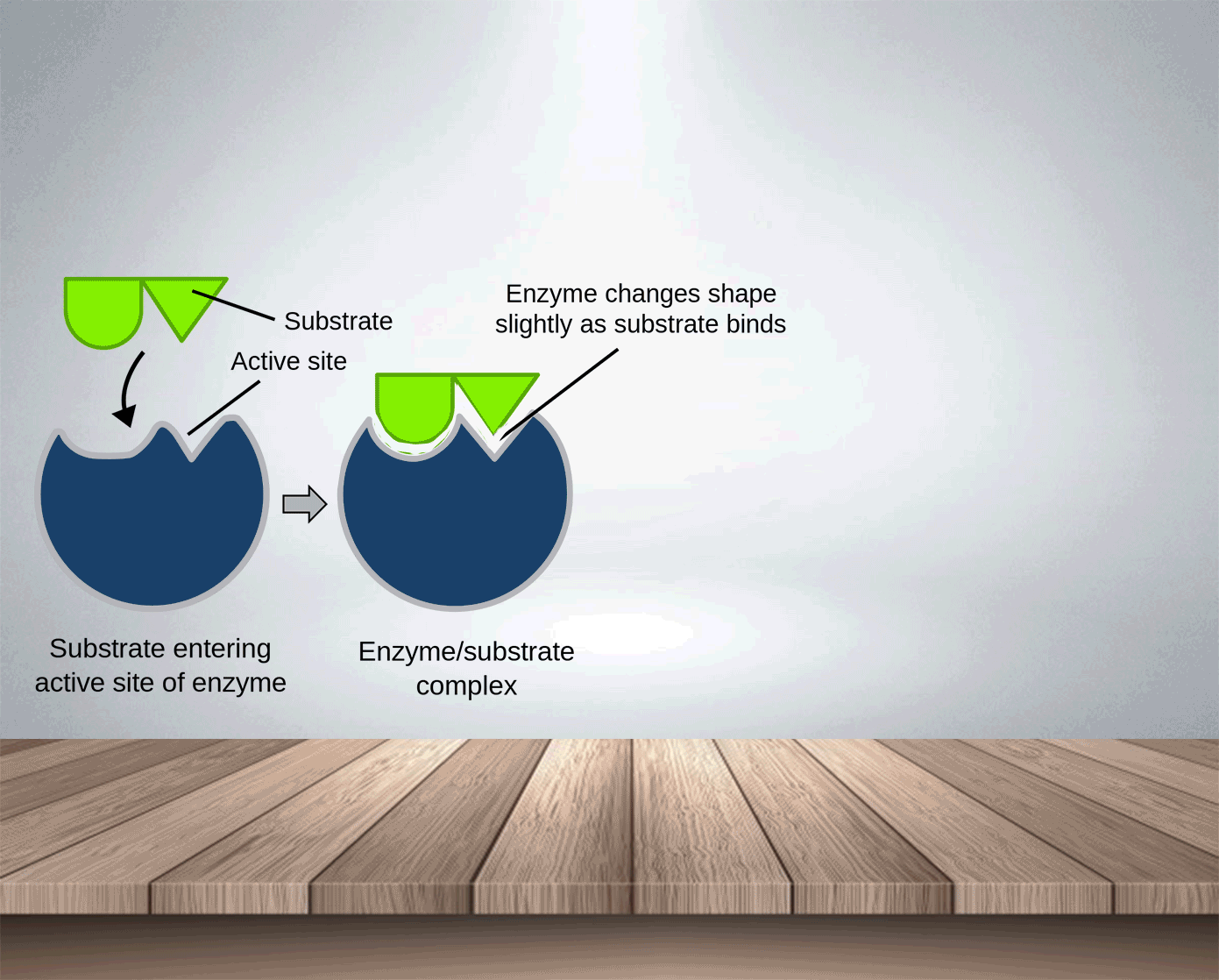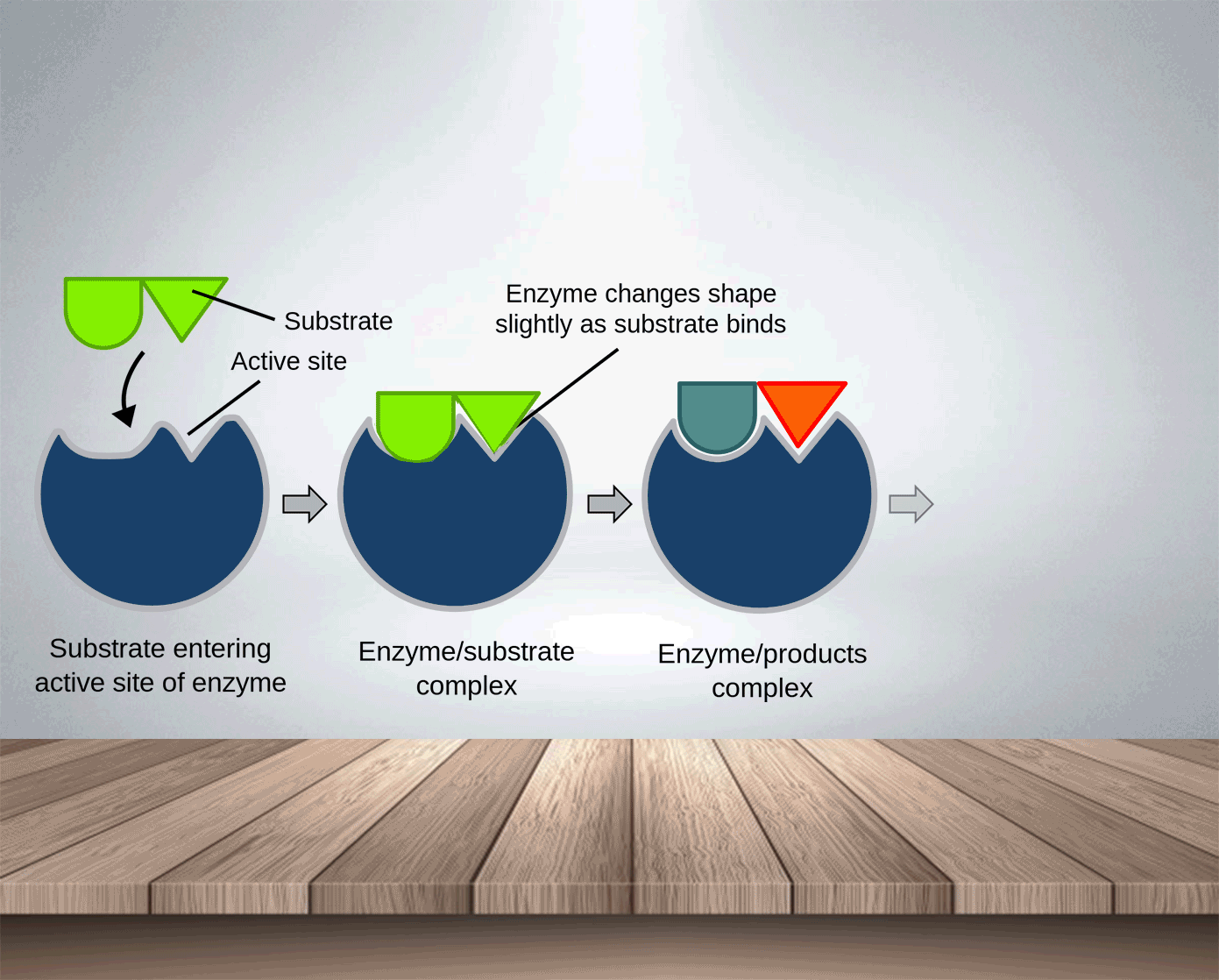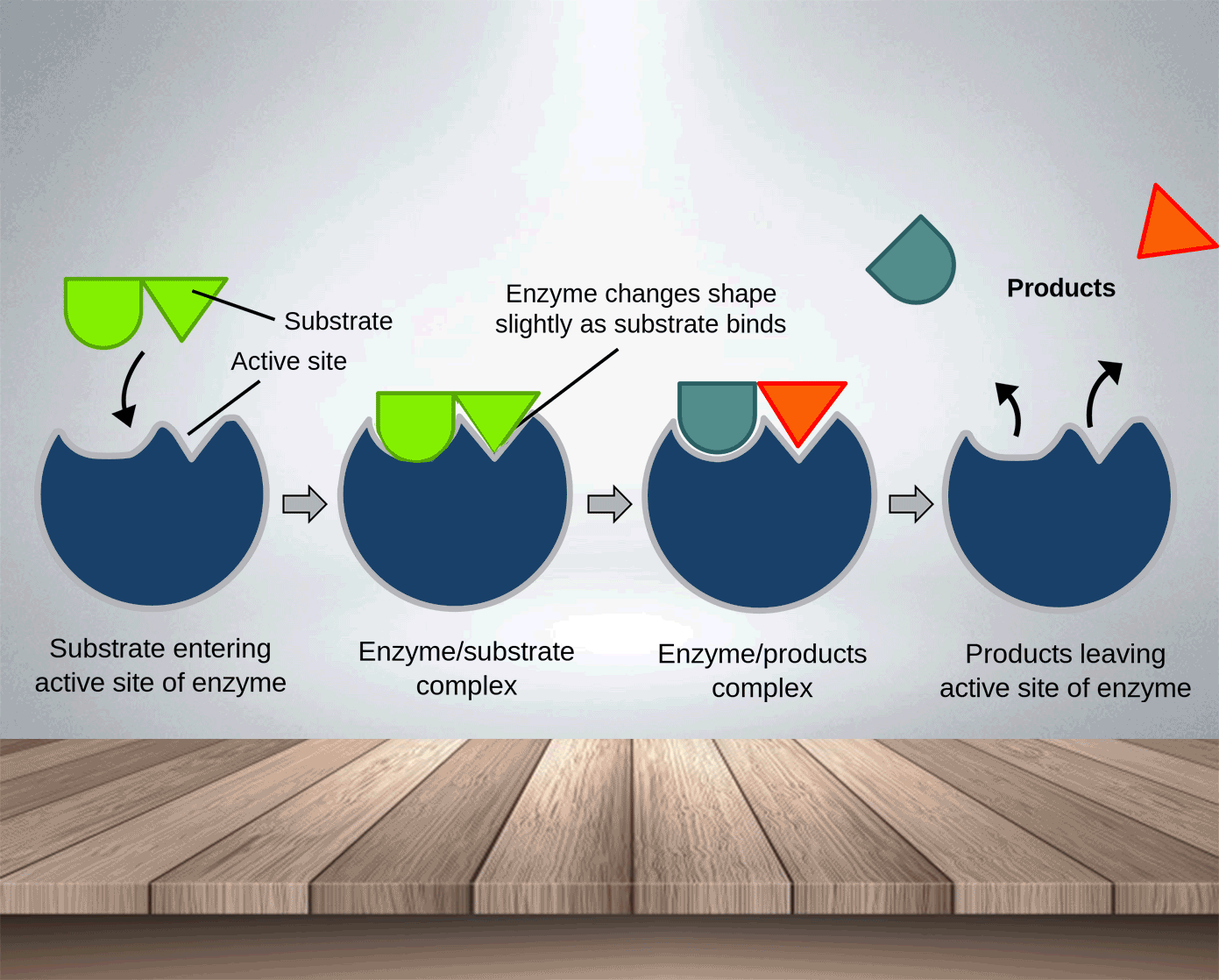
Enzymes, like catalysts, can be used over and over again because they are not used up during the reaction and only a small amount is needed to speed the reaction up. The active side of enzymes exactly fits the substances on which it acts. Thus, substrate molecules and the shape of the active site of the enzyme molecule have complementary shapes (like adjacent pieces of a jigsaw). They can join in an enzyme-substrate complex before forming the products of reation. Hence, enzymes are specific and an enzyme which normally acts on one substance will not act on a different one.
Substrate: the substance on which the enzyme acts.
Products: the molecules produced.
Anabolic reaction: reactions in which large molecules are built up from smaller molecules.
Catabolic reaction: reactions that splits large molecules into smaller ones.
Enzyme-substrate complex: is formed temporarily when the enzyme combines with the substrate.
[Supplement] Property of enzymes
In the lock and key model, the shape of the active site matches the shape of its substrate molecules. Thus,
Enzymes are highly specific : each type of enzyme can catalyse only one type of reaction.
[Supplement] Effect of Temperature
Effect of temperature:
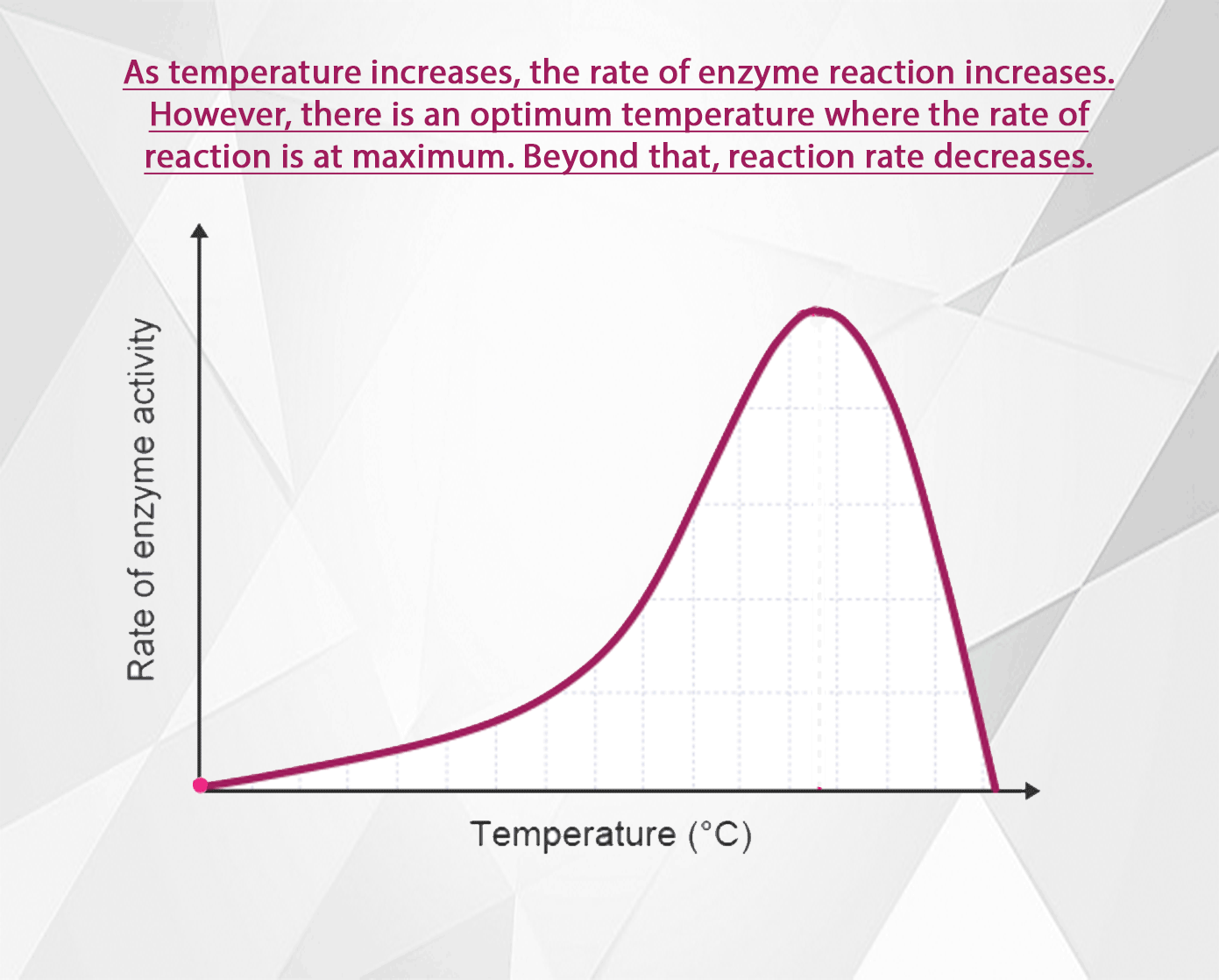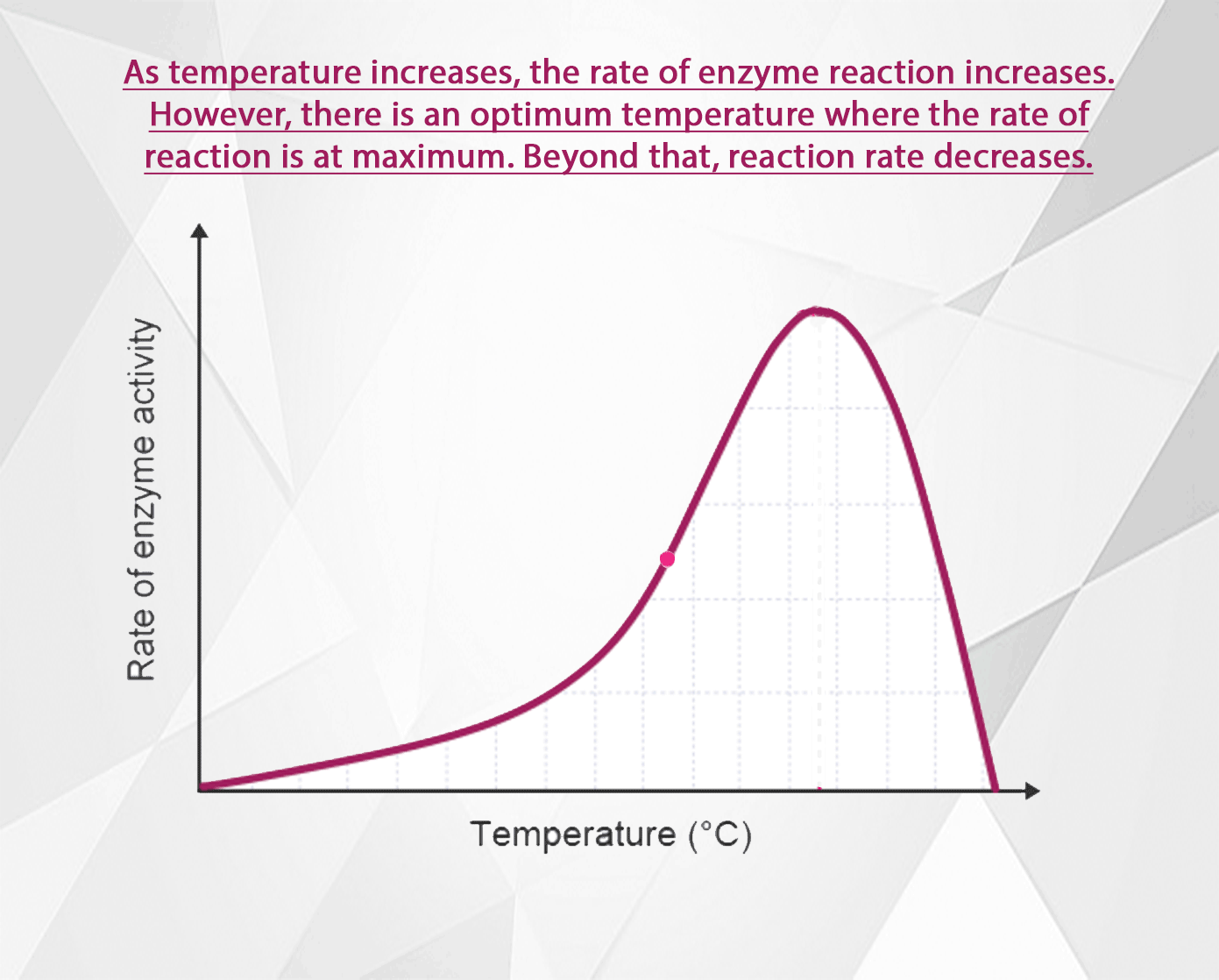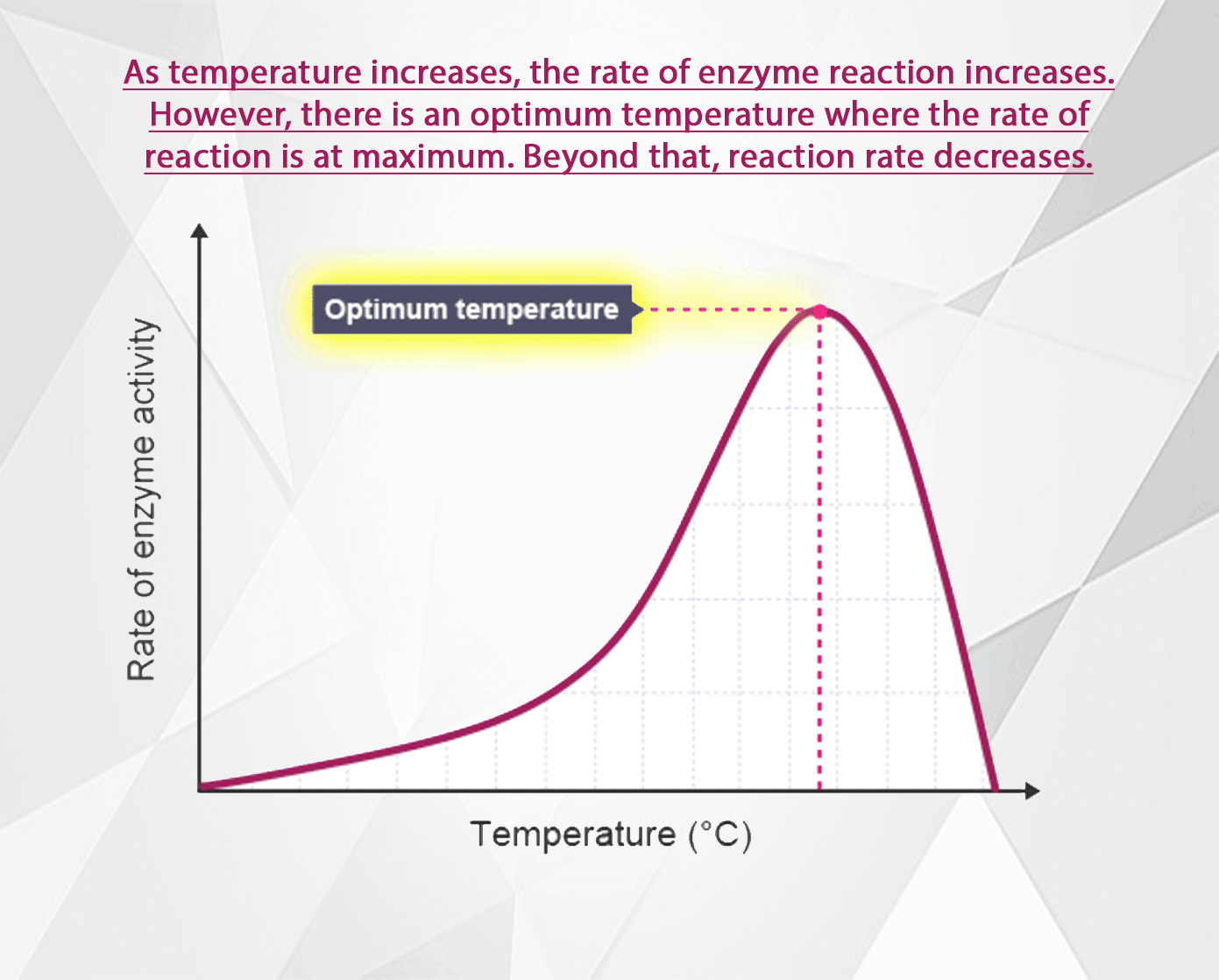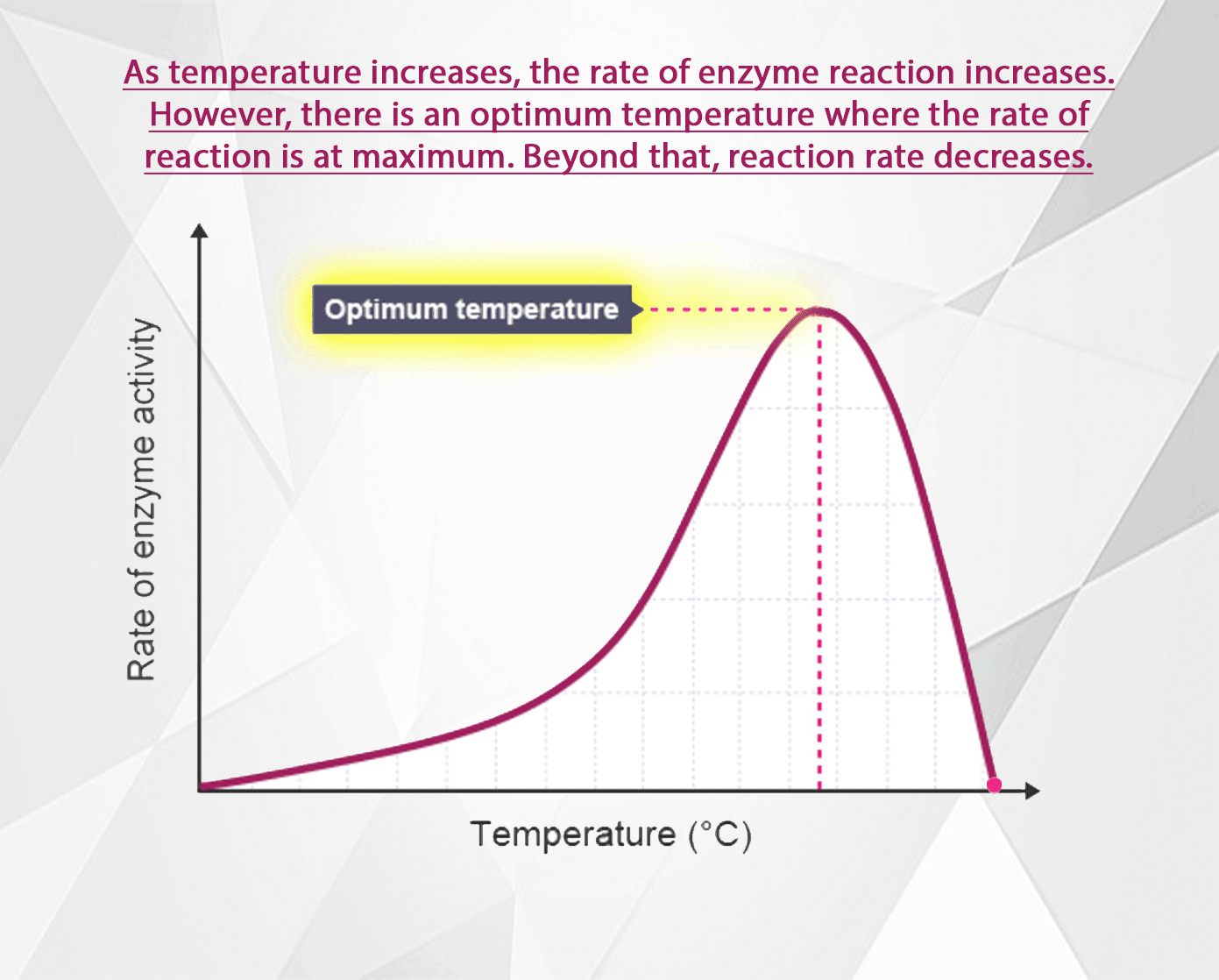
The rate of an enzyme-catalysed reaction increases as the temperature increases. However, at high temperatures the rate decreases again because the enzyme becomes denatured and can no longer function as a biological catalyst.
[Supplement] Effect of Temperature - Video
All molecules are in motion (except at absolute zero). As the temperature increases, their motion increases too. In the case of enzyme catalyzed reactions, as the speed of enzyme and substrate molecules increases, the chance for collisions so they can form enzyme-substrate complexes increases. Thus as the temperature rises, the reaction rate increases too.
Above the optimal temperature however, this does not apply. The reaction rate begins to decrease again because some of the enzyme molecules are now warm enough so that their shape becomes altered (H bonds begin to break, denaturing the enzymes).
As the temperature rises above the optimal then, an increasing number of enzymes become denatured. Fewer and fewer enzymes are able to fit with their substrates at the active site. The reaction rate decreases until at some high temperature, all the enzymes are denatured, and reactions cease.
Effect of Temperature - Description
1. As the temperature is increased, the molecules gain more kinetic energy, so they move faster and there is a greater chance of collisions happening. Therefore the rate of reaction increases. 2. Above the optimum temperature the reaction slows down. This is because enzyme molecules are proteins. Proteins molecules start to lose their shape at higher temperatures, so the active site becomes deformed. 3. Substrate molecules are denatured and cannot fit together with the enzyme, stopping the reaction.
[Supplement] Effect of pH
The pH of a solution is how acidic or alkaline it is.
Different enzymes work best at different pH values.
The optimum pH for an enzyme depends on where it normally works.
It is around neutral (pH 7) for most enzymes but there are some exceptions.
Effect of pH - Description
Changes in pH alter the shape of the active site of an enzyme. Different enzymes work best at different pH values.
The optimum pH for an enzyme depends on its environment. For example, intestinal enzymes have an optimum pH of about 7.5 but stomach enzymes have an optimum pH of about 2.
1. As pH increases/decreases, the active side of an enzyme changes shape. 2. Rate of reaction decreases as less products are formed when the enzymes do not fit the substrates. 3. At extreme pH, enzymes are denatured and the active sites are permanantly denatured.